Template:Psychostimulant addiction
From HandWiki
This annotated image insertion template is intended for transcluding to a variety of psychostimulant and addiction articles, as well as those on related protein topics.
The image file is located at COMMONS:File:ΔFosB.svg and a reusable version of this image is located at COMMONS:File:Annotated ΔFosB.svg screenshot.png.
The image file is located at COMMONS:File:ΔFosB.svg and a reusable version of this image is located at COMMONS:File:Annotated ΔFosB.svg screenshot.png.
References
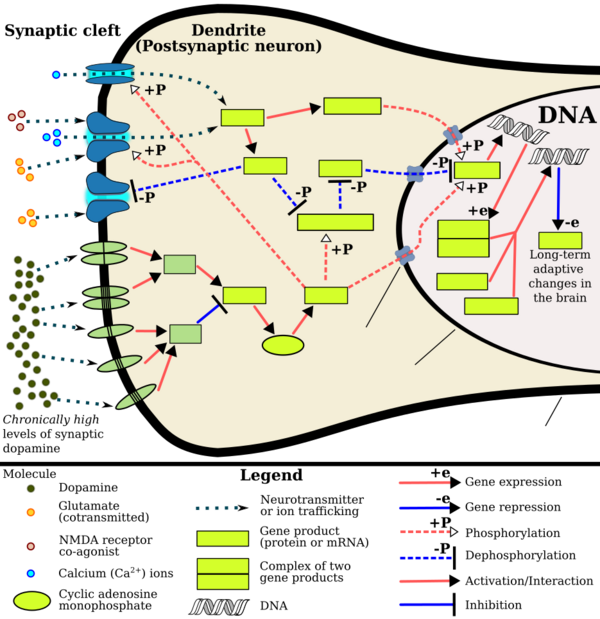
- ↑ 1.0 1.1 1.2 Renthal W, Nestler EJ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues in Clinical Neuroscience 11 (3): 257–268. PMID 19877494. "[Psychostimulants] increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. ΔFosB is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. ... Chronic exposure to psychostimulants increases glutamatergic [signaling] from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5.".
Figure 2: Psychostimulant-induced signaling events - ↑ "Co-transmission of dopamine and glutamate". The Journal of General Physiology 139 (1): 93–96. January 2012. doi:10.1085/jgp.201110659. PMID 22200950. "Coincident and convergent input often induces plasticity on a postsynaptic neuron. The NAc integrates processed information about the environment from basolateral amygdala, hippocampus, and prefrontal cortex (PFC), as well as projections from midbrain dopamine neurons. Previous studies have demonstrated how dopamine modulates this integrative process. For example, high frequency stimulation potentiates hippocampal inputs to the NAc while simultaneously depressing PFC synapses (Goto and Grace, 2005). The converse was also shown to be true; stimulation at PFC potentiates PFC–NAc synapses but depresses hippocampal–NAc synapses. In light of the new functional evidence of midbrain dopamine/glutamate co-transmission (references above), new experiments of NAc function will have to test whether midbrain glutamatergic inputs bias or filter either limbic or cortical inputs to guide goal-directed behavior.".
- ↑ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. http://www.genome.jp/kegg-bin/show_pathway?hsa05031+2354. Retrieved 31 October 2014. "Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. ... Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain."
- ↑ "Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat". Molecular Neurobiology 51 (2): 696–717. 2015. doi:10.1007/s12035-014-8776-8. PMID 24939695. "Figure 1".
- ↑ 5.0 5.1 5.2 Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews Neuroscience 12 (11): 623–637. doi:10.1038/nrn3111. PMID 21989194. "ΔFosB serves as one of the master control proteins governing this structural plasticity. ... ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. ... In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). ... The net result is c-fos gene repression.".
Figure 4: Epigenetic basis of drug regulation of gene expression - ↑ 6.0 6.1 6.2 Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience 10 (3): 136–143. doi:10.9758/cpn.2012.10.3.136. PMID 23430970. "The 35-37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB ... In contrast, the ability of ΔFosB to repress the c-Fos gene occurs in concert with the recruitment of a histone deacetylase and presumably several other repressive proteins such as a repressive histone methyltransferase".
- ↑ Nestler EJ (October 2008). "Transcriptional mechanisms of addiction: Role of ΔFosB". Philosophical Transactions of the Royal Society B: Biological Sciences 363 (1507): 3245–3255. doi:10.1098/rstb.2008.0067. PMID 18640924. "Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure".
- ↑ (Text color) Transcription factors