Physics:Ion source
An ion source is a device that creates atomic and molecular ions.[1] Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Electron ionization
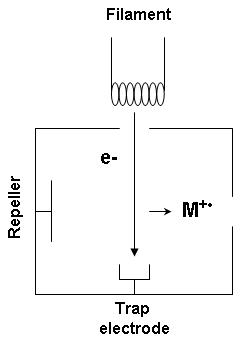
Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is
- [math]\ce{ M{} + e^- -> M^{+\bullet}{} + 2e^- }[/math]
where M is the atom or molecule being ionized, [math]\ce{ e^- }[/math] is the electron, and [math]\ce{ M^{+\bullet} }[/math] is the resulting ion.
The electrons may be created by an arc discharge between a cathode and an anode.
An electron beam ion source (EBIS) is used in atomic physics to produce highly charged ions by bombarding atoms with a powerful electron beam.[2][3] Its principle of operation is shared by the electron beam ion trap.
Electron capture ionization
Electron capture ionization (ECI) is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A−•. The reaction is
- [math]\ce{ A + e^- ->[M] A^- }[/math]
where the M over the arrow denotes that to conserve energy and momentum a third body is required (the molecularity of the reaction is three).
Electron capture can be used in conjunction with chemical ionization.[4]
An electron capture detector is used in some gas chromatography systems.[5]
Chemical ionization
Chemical ionization (CI) is a lower energy process than electron ionization because it involves ion/molecule reactions rather than electron removal.[6] The lower energy yields less fragmentation, and usually a simpler spectrum. A typical CI spectrum has an easily identifiable molecular ion.[7]
In a CI experiment, ions are produced through the collision of the analyte with ions of a reagent gas in the ion source. Some common reagent gases include: methane, ammonia, and isobutane. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization plasma. Positive and negative ions of the analyte are formed by reactions with this plasma. For example, protonation occurs by
- [math]\ce{ CH4 + e^- -> CH4+ + 2e^- }[/math] (primary ion formation),
- [math]\ce{ CH4 + CH4+ -> CH5+ + CH3 }[/math] (reagent ion formation),
- [math]\ce{ M + CH5+ -> CH4 + [M + H]+ }[/math] (product ion formation, e.g. protonation).
Charge exchange ionization
Charge-exchange ionization (also known as charge-transfer ionization) is a gas phase reaction between an ion and an atom or molecule in which the charge of the ion is transferred to the neutral species.[8]
- [math]\ce{ A+ + B -> A + B+ }[/math]
Chemi-ionization
Chemi-ionization is the formation of an ion through the reaction of a gas phase atom or molecule with an atom or molecule in an excited state.[9][10] Chemi-ionization can be represented by
- [math]\ce{ G^\ast{} + M -> G{} + M^{+\bullet}{} + e^- }[/math]
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an electron to form the radical cation (indicated by the superscripted "plus-dot").
Associative ionization
Associative ionization is a gas phase reaction in which two atoms or molecules interact to form a single product ion.[11][12][13] One or both of the interacting species may have excess internal energy.
For example,
- [math]\ce{ A^\ast{} + B -> AB^{+\bullet}{} + e^- }[/math]
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB+.
Penning ionization
Penning ionization is a form of chemi-ionization involving reactions between neutral atoms or molecules.[14][15] The process is named after the Dutch physicist Frans Michel Penning who first reported it in 1927.[16] Penning ionization involves a reaction between a gas-phase excited-state atom or molecule G* and a target molecule M resulting in the formation of a radical molecular cation M+., an electron e−, and a neutral gas molecule G:[17]
- [math]\ce{ G^\ast{} + M -> G{} + M^{+\bullet}{} + e^- }[/math]
Penning ionization occurs when the target molecule has an ionization potential lower than the internal energy of the excited-state atom or molecule.
Associative Penning ionization can proceed via
- [math]\ce{ G^\ast{} + M -> MG^{+\bullet}{} + e^- }[/math]
Surface Penning ionization (also known as Auger deexcitation) refers to the interaction of the excited-state gas with a bulk surface S, resulting in the release of an electron according to
- [math]\ce{ G^\ast{} + S -> G{} + S{} + e^- }[/math].
Ion attachment
Ion-attachment ionization is similar to chemical ionization in which a cation is attached to the analyte molecule in a reactive collision:
- [math]\ce{ M + X+ + A -> MX+ + A }[/math]
Where M is the analyte molecule, X+ is the cation and A is a non-reacting collision partner.[18]
In a radioactive ion source, a small piece of radioactive material, for instance 63Ni or 241Am, is used to ionize a gas.[citation needed] This is used in ionization smoke detectors and ion mobility spectrometers.
Gas-discharge ion sources

These ion sources use a plasma source or electric discharge to create ions.
Inductively-coupled plasma
Ions can be created in an inductively coupled plasma, which is a plasma source in which the energy is supplied by electrical currents which are produced by electromagnetic induction, that is, by time-varying magnetic fields.[19]
Microwave-induced plasma
Microwave induced plasma ion sources are capable of exciting electrodeless gas discharges to create ions for trace element mass spectrometry.[20][21] A microwave plasma is a type of plasma, that has high frequency electromagnetic radiation in the GHz range. It is capable of exciting electrodeless gas discharges. If applied in surface-wave-sustained mode, they are especially well suited to generate large-area plasmas of high plasma density. If they are both in surface-wave and resonator mode, they can exhibit a high degree of spatial localization. This allows to spatially separate the location of plasma generations from the location of surface processing. Such a separation (together with an appropriate gas-flow scheme) may help reduce the negative effect, that particles released from a processed substrate may have on the plasma chemistry of the gas phase.
ECR ion source
The ECR ion source makes use of the electron cyclotron resonance to ionize a plasma. Microwaves are injected into a volume at the frequency corresponding to the electron cyclotron resonance, defined by the magnetic field applied to a region inside the volume. The volume contains a low pressure gas.
Glow discharge
Ions can be created in an electric glow discharge. A glow discharge is a plasma formed by the passage of electric current through a low-pressure gas. It is created by applying a voltage between two metal electrodes in an evacuated chamber containing gas. When the voltage exceeds a certain value, called the striking voltage, the gas forms a plasma.
A duoplasmatron is a type of glow discharge ion source that consists of a cathode (hot cathode or cold cathode) that produces a plasma that is used to ionize a gas.[1][22] Duoplasmatrons can produce positive or negative ions.[23] Duoplasmatrons are used for secondary ion mass spectrometry.,[24][25] ion beam etching, and high-energy physics.[26]
Flowing afterglow
In a flowing afterglow, ions are formed in a flow of inert gas, typically helium or argon.[27][28][29] Reagents are added downstream to create ion products and study reaction rates. Flowing-afterglow mass spectrometry is used for trace gas analysis [30] for organic compounds.[31]
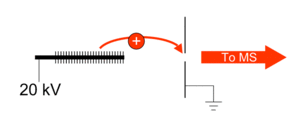
Spark ionization
Electric spark ionization is used to produce gas phase ions from a solid sample. When incorporated with a mass spectrometer the complete instrument is referred to as a spark ionization mass spectrometer or as a spark source mass spectrometer (SSMS).[32]
A closed drift ion source uses a radial magnetic field in an annular cavity in order to confine electrons for ionizing a gas. They are used for ion implantation and for space propulsion (Hall-effect thrusters).
Photoionization
Photoionization is the ionization process in which an ion is formed from the interaction of a photon with an atom or molecule.[33]
Multi-photon ionization
In multi-photon ionization (MPI), several photons of energy below the ionization threshold may actually combine their energies to ionize an atom.
Resonance-enhanced multiphoton ionization (REMPI) is a form of MPI in which one or more of the photons accesses a bound-bound transition that is resonant in the atom or molecule being ionized.
Atmospheric pressure photoionization
Atmospheric pressure photoionization (APPI) uses a source of photons, usually a vacuum UV (VUV) lamp, to ionize the analyte with single photon ionization process. Analogous to other atmospheric pressure ion sources, a spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius) and sprayed with high flow rates of nitrogen for desolvation. The resulting aerosol is subjected to UV radiation to create ions. Atmospheric-pressure laser ionization uses UV laser light sources to ionize the analyte via MPI.
Desorption ionization
Field desorption
Field desorption refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed.[34] This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations [math]\ce{ M^{+.} }[/math] and less often, protonated molecules [math]\ce{ [{M}+H]+ }[/math].
Particle bombardment
Fast atom bombardment
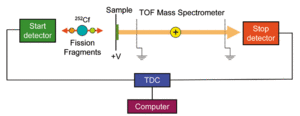
Particle bombardment with atoms is called fast atom bombardment (FAB) and bombardment with atomic or molecular ions is called secondary ion mass spectrometry (SIMS).[35] Fission fragment ionization uses ionic or neutral atoms formed as a result of the nuclear fission of a suitable nuclide, for example the Californium isotope 252Cf.
In FAB the analytes is mixed with a non-volatile chemical protection environment called a matrix and is bombarded under vacuum with a high energy (4000 to 10,000 electron volts) beam of atoms.[36] The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
Secondary ionization
Secondary ion mass spectrometry (SIMS) is used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm.
In a liquid metal ion source (LMIS), a metal (typically gallium) is heated to the liquid state and provided at the end of a capillary or a needle. Then a Taylor cone is formed under the application of a strong electric field. As the cone's tip get sharper, the electric field becomes stronger, until ions are produced by field evaporation. These ion sources are particularly used in ion implantation or in focused ion beam instruments.
Plasma desorption ionization
Plasma desorption ionization mass spectrometry (PDMS), also called fission fragment ionization, is a mass spectrometry technique in which ionization of material in a solid sample is accomplished by bombarding it with ionic or neutral atoms formed as a result of the nuclear fission of a suitable nuclide, typically the californium isotope 252Cf.[37][38]
Laser desorption ionization
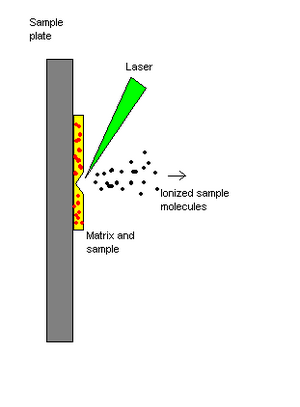
Matrix-assisted laser desorption/ionization (MALDI) is a soft ionization technique. The sample is mixed with a matrix material. Upon receiving a laser pulse, the matrix absorbs the laser energy and it is thought that primarily the matrix is desorbed and ionized (by addition of a proton) by this event. The analyte molecules are also desorbed. The matrix is then thought to transfer proton to the analyte molecules (e.g., protein molecules), thus charging the analyte.
Surface-assisted laser desorption/ionization
Surface-assisted laser desorption/ionization (SALDI) is a soft laser desorption technique used for analyzing biomolecules by mass spectrometry.[39][40] In its first embodiment, it used graphite matrix.[39] At present, laser desorption/ionization methods using other inorganic matrices, such as nanomaterials, are often regarded as SALDI variants. A related method named "ambient SALDI" - which is a combination of conventional SALDI with ambient mass spectrometry incorporating the DART ion source - has also been demonstrated.[41]
Surface-enhanced laser desorption/ionization
Surface-enhanced laser desorption/ionization (SELDI) is a variant of MALDI that is used for the analysis of protein mixtures that uses a target modified to achieve biochemical affinity with the analyte compound.[42]
Desorption ionization on silicon
Desorption ionization on silicon (DIOS) refers to laser desorption/ionization of a sample deposited on a porous silicon surface.[43]
Smalley source
A laser vaporization cluster source produces ions using a combination of laser desorption ionization and supersonic expansion.[44] The Smalley source (or Smalley cluster source)[45] was developed by Richard Smalley at Rice University in the 1980s and was central to the discovery of fullerenes in 1985.[46][47]
Aerosol ionization
In aerosol mass spectrometry with time-of-flight analysis, micrometer sized solid aerosol particles extracted from the atmosphere are simultaneously desorbed and ionized by a precisely timed laser pulse as they pass through the center of a time-of-flight ion extractor.[48][49]
Spray ionization
Spray ionization methods involve the formation of aerosol particles from a liquid solution and the formation of bare ions after solvent evaporation.[50]
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI.[51] Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity.[52] The inlet tube to the mass spectrometer becomes the ion source.
Matrix-Assisted Ionization
Matrix-Assisted Ionization [MAI] is similar to MALDI in sample preparation, but a laser is not required to convert analyte molecules included in a matrix compound into gas-phase ions. In MAI, analyte ions have charge states similar to electrospray ionization but obtained from a solid matrix rather than a solvent. No voltage or laser is required, but a laser can be used to obtain spatial resolution for imaging. Matrix-analyte samples are ionized in the vacuum of a mass spectrometer and can be inserted into the vacuum through an atmospheric pressure inlet. Less volatile matrices such as 2,5-dihydroxybenzoic acid require a hot inlet tube to produce analyte ions by MAI, but more volatile matrices such as 3-nitrobenzonitrile require no heat, voltage, or laser. Simply introducing the matrix:analyte sample to the inlet aperture of an atmospheric pressure ionization mass spectrometer produces abundant ions. Compounds at least as large as bovine serum albumin [66 kDa] can be ionized with this method.[53] In this simple, low cost and easy to use ionization method, the inlet to the mass spectrometer can be considered the ion source.
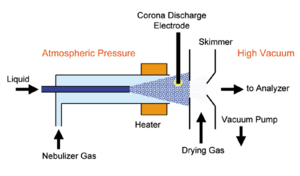
Atmospheric-pressure chemical ionization
Atmospheric-pressure chemical ionization is a form of chemical ionization using a solvent spray at atmospheric pressure.[54] A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen and the entire aerosol cloud is subjected to a corona discharge that creates ions with the evaporated solvent acting as the chemical ionization reagent gas. APCI is not as "soft" (low fragmentation) an ionization technique as ESI.[55] Note that atmospheric pressure ionization (API) should not be used as a synonym for APCI.[56]
Thermospray ionization
Thermospray ionization is a form of atmospheric pressure ionization in mass spectrometry. It transfers ions from the liquid phase to the gas phase for analysis. It is particularly useful in liquid chromatography-mass spectrometry.[57]
Electrospray ionization
In electrospray ionization, a liquid is pushed through a very small, charged and usually metal, capillary.[58] This liquid contains the substance to be studied, the analyte, dissolved in a large amount of solvent, which is usually much more volatile than the analyte. Volatile acids, bases or buffers are often added to this solution too. The analyte exists as an ion in solution either in its anion or cation form. Because like charges repel, the liquid pushes itself out of the capillary and forms an aerosol, a mist of small droplets about 10 μm across. The aerosol is at least partially produced by a process involving the formation of a Taylor cone and a jet from the tip of this cone. An uncharged carrier gas such as nitrogen is sometimes used to help nebulize the liquid and to help evaporate the neutral solvent in the droplets. As the solvent evaporates, the analyte molecules are forced closer together, repel each other and break up the droplets. This process is called Coulombic fission because it is driven by repulsive Coulombic forces between charged molecules. The process repeats until the analyte is free of solvent and is a bare ion. The ions observed are created by the addition of a proton (a hydrogen ion) and denoted [math]\ce{ [{M}+H]+ }[/math], or of another cation such as sodium ion, [math]\ce{ [M + Na]+ }[/math], or the removal of a proton, [math]\ce{ [M - H]^- }[/math]. Multiply charged ions such as [math]\ce{ [{M}+2H]^2+ }[/math] are often observed. For large macromolecules, there can be many charge states, occurring with different frequencies; the charge can be as great as [math]\ce{ [M + 25H]^{25+} }[/math], for example.
Probe electrospray ionization
Probe electrospray ionization (PESI) is a modified version of electrospray, where the capillary for sample solution transferring is replaced by a sharp-tipped solid needle with periodical motion.[59]
Contactless atmospheric pressure ionization
Contactless atmospheric pressure ionization is a technique used for analysis of liquid and solid samples by mass spectrometry.[60] Contactless API can be operated without an additional electric power supply (supplying voltage to the source emitter), gas supply, or syringe pump. Thus, the technique provides a facile means for analyzing chemical compounds by mass spectrometry at atmospheric pressure.
Sonic spray ionization
Sonic spray ionization is method for creating ions from a liquid solution, for example, a mixture of methanol and water.[61] A pneumatic nebulizer is used to turn the solution into a supersonic spray of small droplets. Ions are formed when the solvent evaporates and the statistically unbalanced charge distribution on the droplets leads to a net charge and complete desolvation results in the formation of ions. Sonic spray ionization is used to analyze small organic molecules and drugs and can analyze large molecules when an electric field is applied to the capillary to help increase the charge density and generate multiple charged ions of proteins.[62]
Sonic spray ionization has been coupled with high performance liquid chromatography for the analysis of drugs.[63][64] Oligonucleotides have been studied with this method.[65][66] SSI has been used in a manner similar to desorption electrospray ionization[67] for ambient ionization and has been coupled with thin-layer chromatography in this manner.[68]
Ultrasonication-assisted spray ionization
Ultrasonication-assisted spray ionization (UASI) involves ionization through the application of ultrasound.[69][70]
Thermal ionization
Thermal ionization (also known as surface ionization, or contact ionization) involves spraying vaporized, neutral atoms onto a hot surface, from which the atoms re-evaporate in ionic form. To generate positive ions, the atomic species should have a low ionization energy, and the surface should have a high work function. This technique is most suitable for alkali atoms (Li, Na, K, Rb, Cs) which have low ionization energies and are easily evaporated.[71]
To generate negative ions, the atomic species should have a high electron affinity, and the surface should have a low work function. This second approach is most suited for halogen atoms Cl, Br, I, At.[72]
Ambient ionization
In ambient ionization, ions are formed outside the mass spectrometer without sample preparation or separation.[73][74][75] Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface.[74][76] Molecules on the surface are extracted into the solvent. The action of the primary droplets hitting the surface produces secondary droplets that are the source of ions for the mass spectrometer. Desorption electrospray ionization (DESI) uses an electrospray source to create charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.[77]
Plasma-based ambient ionization is based on an electrical discharge in a flowing gas that produces metastable atoms and molecules and reactive ions. Heat is often used to assist in the desorption of volatile species from the sample. Ions are formed by chemical ionization in the gas phase. A direct analysis in real time source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral atoms or molecules (or "metastables"). Excited states are typically formed in the DART source by creating a glow discharge in a chamber through which the gas flows. A similar method called atmospheric solids analysis probe [ASAP] uses the heated gas from ESI or APCI probes to vaporize sample placed on a melting point tube inserted into an ESI/APCI source.[78] Ionization is by APCI.
Laser-based ambient ionization is a two-step process in which a pulsed laser is used to desorb or ablate material from a sample and the plume of material interacts with an electrospray or plasma to create ions. Electrospray-assisted laser desorption/ionization (ELDI) uses a 337 nm UV laser[79] or 3 μm infrared laser[80] to desorb material into an electrospray source. Matrix-assisted laser desorption electrospray ionization (MALDESI)[81] is an atmospheric pressure ionization source for generation of multiply charged ions. An ultraviolet or infrared laser is directed onto a solid or liquid sample containing the analyte of interest and matrix desorbing neutral analyte molecules that are ionized by interaction with electrosprayed solvent droplets generating multiply charged ions. Laser ablation electrospray ionization (LAESI) is an ambient ionization method for mass spectrometry that combines laser ablation from a mid-infrared (mid-IR) laser with a secondary electrospray ionization (ESI) process.
Applications
Mass spectrometry
In a mass spectrometer a sample is ionized in an ion source and the resulting ions are separated by their mass-to-charge ratio. The ions are detected and the results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio. The atoms or molecules in the sample can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern.
Particle accelerators


In particle accelerators an ion source creates a particle beam at the beginning of the machine, the source. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated: electrons, protons, H− ion or a Heavy ions.
Electrons are generated with an electron gun, of which there are many varieties.
Protons are generated with a plasma-based device, like a duoplasmatron or a magnetron.
H− ions are generated with a magnetron or a Penning source. A magnetron consists of a central cylindrical cathode surrounded by an anode. The discharge voltage is typically greater than 150 V and the current drain is around 40 A. A magnetic field of about 0.2 tesla is parallel to the cathode axis. Hydrogen gas is introduced by a pulsed gas valve. Caesium is often used to lower the work function of the cathode, enhancing the amount of ions that are produced. Large caesiated H− sources are also used for plasma heating in nuclear fusion devices.
For a Penning source, a strong magnetic field parallel to the electric field of the sheath guides electrons and ions on cyclotron spirals from cathode to cathode. Fast H-minus ions are generated at the cathodes as in the magnetron. They are slowed down due to the charge exchange reaction as they migrate to the plasma aperture. This makes for a beam of ions that is colder than the ions obtained from a magnetron.
Heavy ions can be generated with an electron cyclotron resonance ion source. The use of electron cyclotron resonance (ECR) ion sources for the production of intense beams of highly charged ions has immensely grown over the last decade. ECR ion sources are used as injectors into linear accelerators, Van-de-Graaff generators or cyclotrons in nuclear and elementary particle physics. In atomic and surface physics ECR ion sources deliver intense beams of highly charged ions for collision experiments or for the investigation of surfaces. For the highest charge states, however, Electron beam ion sources (EBIS) are needed. They can generate even bare ions of mid-heavy elements. The Electron beam ion trap (EBIT), based on the same principle, can produce up to bare uranium ions and can be used as an ion source as well.
Heavy ions can also be generated with an ion gun which typically uses the thermionic emission of electrons to ionize a substance in its gaseous state. Such instruments are typically used for surface analysis.
Gas flows through the ion source between the anode and the cathode. A positive voltage is applied to the anode. This voltage, combined with the high magnetic field between the tips of the internal and external cathodes allow a plasma to start. Ions from the plasma are repelled by the anode electric field. This creates an ion beam.[83]
Surface modification
- Surface cleaning and pretreatment for large area deposition
- Thin film deposition
- Deposition of thick diamond-like carbon (DLC) films
- Surface roughening of polymers for improved adhesion and/or biocompatibility[84]
See also
References
- ↑ 1.0 1.1 Bernhard Wolf (31 August 1995). Handbook of Ion Sources. CRC Press. ISBN 978-0-8493-2502-1. https://books.google.com/books?id=qoQHMSBNFsAC.
- ↑ Ian G. Brown (6 March 2006). The Physics and Technology of Ion Sources. John Wiley & Sons. ISBN 978-3-527-60454-8. https://books.google.com/books?id=cdjGzG_zBLwC.
- ↑ Heinrich Beyer; Heinrich F. Beyer; H.-Jürgen Kluge; H.-J. Kluge; Vi͡acheslav Petrovich Shevelʹko (14 August 1997). X-Ray Radiation of Highly Charged Ions. Springer Science & Business Media. ISBN 978-3-540-63185-9. https://books.google.com/books?id=8_AJFBC2-BsC.
- ↑ Donald F. Hunt; Frank W. Crow (1978), "Electron capture negative ion chemical ionization mass spectrometry", Analytical Chemistry 50 (13): 1781–1784, doi:10.1021/ac50035a017
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "electron capture detector (in gas chromatography)". doi:10.1351/goldbook.E01981
- ↑ Munson, M. S. B.; Field, F. H. (1966). "Chemical Ionization Mass Spectrometry. I. General Introduction". Journal of the American Chemical Society 88 (12): 2621–2630. doi:10.1021/ja00964a001.
- ↑ de Hoffmann, Edmond; Vincent Stroobant (2003). Mass Spectrometry: Principles and Applications (Second ed.). Toronto: John Wiley & Sons, Ltd.. p. 14. ISBN 978-0-471-48566-7.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "charge-exchange ionization". doi:10.1351/goldbook.C00989
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chemi-ionization". doi:10.1351/goldbook.C01044 C01044
- ↑ Klucharev, A. N. (1993), "Chemi-ionization processes", Physics-Uspekhi 36 (6): 486–512, doi:10.1070/PU1993v036n06ABEH002162, Bibcode: 1993PhyU...36..486K
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "associative ionization". doi:10.1351/goldbook.A00475
- ↑ *"Theory of associative ionization". Physical Review A 37 (8): 2916–2933. April 1988. doi:10.1103/PhysRevA.37.2916. PMID 9900022. Bibcode: 1988PhRvA..37.2916J.
- ↑ Cohen, James S. (1976). "Multistate curve-crossing model for scattering: Associative ionization and excitation transfer in helium". Physical Review A 13 (1): 99–114. doi:10.1103/PhysRevA.13.99. Bibcode: 1976PhRvA..13...99C.
- ↑ "Cold atomic collisions: coherent control of penning and associative ionization". Phys. Rev. Lett. 97 (19): 193202. 2006. doi:10.1103/PhysRevLett.97.193202. PMID 17155624. Bibcode: 2006PhRvL..97s3202A.
- ↑ "Atmospheric-pressure Penning ionization of aliphatic hydrocarbons". Rapid Commun. Mass Spectrom. 20 (21): 3213–22. 2006. doi:10.1002/rcm.2706. PMID 17016831. Bibcode: 2006RCMS...20.3213H.
- ↑ Penning, F. M. Die Naturwissenschaften, 1927, 15, 818. Über Ionisation durch metastabile Atome.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Penning gas mixture". doi:10.1351/goldbook.P04476
- ↑ Selvin, P. Christopher; Fujii, Toshihiro (2001). "Lithium ion attachment mass spectrometry: Instrumentation and features". Review of Scientific Instruments 72 (5): 2248. doi:10.1063/1.1362439. Bibcode: 2001RScI...72.2248S.
- ↑ A. Montaser and D. W. Golightly, eds. Inductively Coupled Plasmas in Analytical Atomic Spectrometry, VCH Publishers, Inc., New York, 1992.
- ↑ Okamoto, Yukio (1994). "High-sensitivity microwave-induced plasma mass spectrometry for trace element analysis". Journal of Analytical Atomic Spectrometry 9 (7): 745. doi:10.1039/ja9940900745. ISSN 0267-9477.
- ↑ Douglas, D. J.; French, J. B. (1981). "Elemental analysis with a microwave-induced plasma/quadrupole mass spectrometer system". Analytical Chemistry 53 (1): 37–41. doi:10.1021/ac00224a011. ISSN 0003-2700.
- ↑ Lejeune, C. (1974). "Theoretical and experimental study of the duoplasmatron ion source". Nuclear Instruments and Methods 116 (3): 417–428. doi:10.1016/0029-554X(74)90821-0. ISSN 0029-554X. Bibcode: 1974NucIM.116..417L.
- ↑ Aberth, William; Peterson, James R. (1967). "Characteristics of a Low Energy Duoplasmatron Negative Ion Source". Review of Scientific Instruments 38 (6): 745. doi:10.1063/1.1720882. ISSN 0034-6748. Bibcode: 1967RScI...38..745A.
- ↑ Coath, C. D.; Long, J. V. P. (1995). "A high-brightness duoplasmatron ion source for microprobe secondary-ion mass spectrometry". Review of Scientific Instruments 66 (2): 1018. doi:10.1063/1.1146038. ISSN 0034-6748. Bibcode: 1995RScI...66.1018C.
- ↑ Christine M. Mahoney (9 April 2013). Cluster Secondary Ion Mass Spectrometry: Principles and Applications. John Wiley & Sons. pp. 65–. ISBN 978-1-118-58925-0. https://books.google.com/books?id=hVSqwK0uqfsC&pg=PA65.
- ↑ Stanley Humphries (25 July 2013). Charged Particle Beams. Dover Publications. pp. 309–. ISBN 978-0-486-31585-0. https://books.google.com/books?id=1GjCAgAAQBAJ&pg=PA309.
- ↑ Ferguson, E. E.; Fehsenfeld, F. C.; Schmeltekopf, A. L. (1969). "Ion-Molecule Reaction Rates Measured in a Discharge Afterglow". Chemical Reactions in Electrical Discharges. Advances in Chemistry. 80. pp. 83–91. doi:10.1021/ba-1969-0080.ch006. ISBN 978-0-8412-0081-4.
- ↑ Ferguson, Eldon E. (1992). "A Personal history of the early development of the flowing afterglow technique for ion-molecule reaction studies". Journal of the American Society for Mass Spectrometry 3 (5): 479–486. doi:10.1016/1044-0305(92)85024-E. ISSN 1044-0305. PMID 24234490. https://zenodo.org/record/1258658.
- ↑ Bierbaum, Veronica M. (2014). "Go with the flow: Fifty years of innovation and ion chemistry using the flowing afterglow". International Journal of Mass Spectrometry 377: 456–466. doi:10.1016/j.ijms.2014.07.021. ISSN 1387-3806. Bibcode: 2015IJMSp.377..456B.
- ↑ Smith, David; Španěl, Patrik (2005). "Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis". Mass Spectrometry Reviews 24 (5): 661–700. doi:10.1002/mas.20033. ISSN 0277-7037. PMID 15495143. Bibcode: 2005MSRv...24..661S.
- ↑ Dhooghe, Frederik; Vansintjan, Robbe; Schoon, Niels; Amelynck, Crist (2012-08-30). "Studies in search of selective detection of isomeric biogenic hexen-1-ols and hexanal by flowing afterglow tandem mass spectrometry using [H3O]+ and [NO]+ reagent ions" (in en). Rapid Communications in Mass Spectrometry 26 (16): 1868–1874. doi:10.1002/rcm.6294. ISSN 1097-0231. PMID 22777789.
- ↑ H. E. Beske; A. Hurrle; K. P. Jochum (1981). "Part I. Principles of spark source mass spectrometry (SSMS)". Fresenius' Journal of Analytical Chemistry 309 (4): 258–261. doi:10.1007/BF00488596.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "photoionization". doi:10.1351/goldbook.P04620
- ↑ Beckey, H.D. (1969). "Field ionization mass spectrometry". Research/Development 20 (11): 26.
- ↑ Williams, Dudley H.; Findeis, A. Frederick; Naylor, Stephen; Gibson, Bradford W. (1987). "Aspects of the production of FAB and SIMS mass spectra". Journal of the American Chemical Society 109 (7): 1980–1986. doi:10.1021/ja00241a013. ISSN 0002-7863.
- ↑ "Fast atom bombardment: a new mass spectrometric method for peptide sequence analysis". Biochem. Biophys. Res. Commun. 101 (2): 623–31. 1981. doi:10.1016/0006-291X(81)91304-8. PMID 7306100.
- ↑ Macfarlane, R.; Torgerson, D. (1976). "Californium-252 plasma desorption mass spectroscopy". Science 191 (4230): 920–925. doi:10.1126/science.1251202. ISSN 0036-8075. PMID 1251202. Bibcode: 1976Sci...191..920M.
- ↑ Hilf, E.R. (1993). "Approaches to plasma desorption mass spectrometry by some theoretical physics concepts". International Journal of Mass Spectrometry and Ion Processes 126: 25–36. doi:10.1016/0168-1176(93)80067-O. ISSN 0168-1176. Bibcode: 1993IJMSI.126...25H.
- ↑ 39.0 39.1 Sunner, Jan.; Dratz, Edward.; Chen, Yu-Chie. (1995). "Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions". Analytical Chemistry 67 (23): 4335–4342. doi:10.1021/ac00119a021. ISSN 0003-2700. PMID 8633776.
- ↑ Dattelbaum, Andrew M; Iyer, Srinivas (2006). "Surface-assisted laser desorption/ionization mass spectrometry". Expert Review of Proteomics 3 (1): 153–161. doi:10.1586/14789450.3.1.153. ISSN 1478-9450. PMID 16445359. https://zenodo.org/record/1235756.
- ↑ Zhang, Jialing; Li, Ze; Zhang, Chengsen; Feng, Baosheng; Zhou, Zhigui; Bai, Yu; Liu, Huwei (2012). "Graphite-Coated Paper as Substrate for High Sensitivity Analysis in Ambient Surface-Assisted Laser Desorption/Ionization Mass Spectrometry". Analytical Chemistry 84 (7): 3296–3301. doi:10.1021/ac300002g. ISSN 0003-2700. PMID 22380704.
- ↑ "Current developments in SELDI affinity technology". Mass Spectrometry Reviews 23 (1): 34–44. 2004. doi:10.1002/mas.10066. PMID 14625891. Bibcode: 2004MSRv...23...34T.
- ↑ Buriak, Jillian M.; Wei, Jing; Siuzdak, Gary (1999). "Desorption-ionization mass spectrometry on porous silicon". Nature 399 (6733): 243–246. doi:10.1038/20400. ISSN 0028-0836. PMID 10353246. Bibcode: 1999Natur.399..243W.
- ↑ Duncan, Michael A. (2012). "Invited Review Article: Laser vaporization cluster sources". Review of Scientific Instruments 83 (4): 041101–041101–19. doi:10.1063/1.3697599. ISSN 0034-6748. PMID 22559508. Bibcode: 2012RScI...83d1101D.
- ↑ Laser Ablation and Desorption. Academic Press. 10 December 1997. pp. 628–. ISBN 978-0-08-086020-6. https://books.google.com/books?id=iWfddcv0quwC&pg=PA628.
- ↑ Smalley, Richard (1997). "Discovering the fullerenes". Reviews of Modern Physics 69 (3): 723–730. doi:10.1103/RevModPhys.69.723. ISSN 0034-6861. Bibcode: 1997RvMP...69..723S.
- ↑ Roy L. Johnston (25 April 2002). Atomic and Molecular Clusters. CRC Press. pp. 150–. ISBN 978-1-4200-5577-1. https://books.google.com/books?id=pxztbPhmBeIC&pg=PA150.
- ↑ Carson, P; Neubauer, K; Johnston, M; Wexler, A (1995). "On-line chemical analysis of aerosols by rapid single-particle mass spectrometry". Journal of Aerosol Science 26 (4): 535–545. doi:10.1016/0021-8502(94)00133-J. Bibcode: 1995JAerS..26..535C.
- ↑ Guazzotti, S; Coffee, K; Prather, K (2000). "Real time monitoring of size-resolved single particle chemistry during INDOEX-IFP 99". Journal of Aerosol Science 31: 182–183. doi:10.1016/S0021-8502(00)90189-7. Bibcode: 2000JAerS..31..182G.
- ↑ Chhabil Dass (11 May 2007). Fundamentals of Contemporary Mass Spectrometry. John Wiley & Sons. pp. 45–57. ISBN 978-0-470-11848-1. https://books.google.com/books?id=CYx9wzBzlIsC.
- ↑ "Solvent Assisted Inlet Ionization: an Ultrasensitive New Liquid Introduction Ionization Method for Mass Spectrometry". Anal. Chem. 83 (11): 3981–3985. 2011. doi:10.1021/ac200556z. PMID 21528896.
- ↑ "Increasing the Sensitivity of Liquid Introduction Mass Spectrometry by Combining Electrospray Ionization and Solvent Assisted Inlet Ionization". Anal. Chem. 84 (15): 6828–6832. 2012. doi:10.1021/ac3014115. PMID 22742705.
- ↑ "New Ionization Processes and Applications for Use in Mass Spectrometry". Rev. Biochem. Mol. Biol. 2013 5: 409–429.
- ↑ "Analytical strategies for identifying drug metabolites". Mass Spectrometry Reviews 26 (3): 340–69. 2007. doi:10.1002/mas.20128. PMID 17405144. Bibcode: 2007MSRv...26..340P.
- ↑ "Derivatization in mass spectrometry--8. Soft ionization mass spectrometry of small molecules". European Journal of Mass Spectrometry 12 (2): 79–115. 2006. doi:10.1255/ejms.798. PMID 16723751.
- ↑ "Atmospheric pressure ionization in mass spectrometry". IUPAC Compendium of Chemical Terminology. 2009. doi:10.1351/goldbook.A00492. ISBN 978-0-9678550-9-7.
- ↑ Blakley, C. R.; Carmody, J. J.; Vestal, M. L. (1980). "Liquid Chromatograph-Mass Spectrometer for Analysis of Nonvolatile Samples". Analytical Chemistry 1980 (52): 1636–1641. doi:10.1021/ac50061a025.
- ↑ Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. (1990). "Electrospray Ionization-Principles and Practice". Mass Spectrometry Reviews 9 (1): 37–70. doi:10.1002/mas.1280090103. Bibcode: 1990MSRv....9...37F.
- ↑ Hiraoka K.; Nishidate K.; Mori K.; Asakawa D.; Suzuki S. (2007). "Development of probe electrospray using a solid needle". Rapid Communications in Mass Spectrometry 21 (18): 3139–3144. doi:10.1002/rcm.3201. PMID 17708527. Bibcode: 2007RCMS...21.3139H.
- ↑ Hsieh, Cheng-Huan; Chang, Chia-Hsien; Urban, Pawel L.; Chen, Yu-Chie (2011). "Capillary Action-Supported Contactless Atmospheric Pressure Ionization for the Combined Sampling and Mass Spectrometric Analysis of Biomolecules". Analytical Chemistry 83 (8): 2866–2869. doi:10.1021/ac200479s. ISSN 0003-2700. PMID 21446703.
- ↑ "Sonic spray mass spectrometry". Anal. Chem. 67 (17): 2878–82. 1995. doi:10.1021/ac00113a023. PMID 8779414.
- ↑ Chen, Tsung-Yi; Lin, Jia-Yi; Chen, Jen-Yi; Chen, Yu-Chie (2011-11-22). "Ultrasonication-assisted spray ionization mass spectrometry for the analysis of biomolecules in solution" (in en). Journal of the American Society for Mass Spectrometry 21 (9): 1547–1553. doi:10.1016/j.jasms.2010.04.021. ISSN 1044-0305. PMID 20547459.
- ↑ "Comparison of SSI with APCI as an interface of HPLC-mass spectrometry for analysis of a drug and its metabolites". J. Am. Soc. Mass Spectrom. 13 (3): 204–208. 2002. doi:10.1016/S1044-0305(01)00359-2. PMID 11908800.
- ↑ "Sonic spray ionization technology: performance study and application to a LC/MS analysis on a monolithic silica column for heroin impurity profiling". Anal. Chem. 74 (13): 3206–3212. 2002. doi:10.1021/ac0112824. PMID 12141684.
- ↑ "Matrix effect on the analysis of oligonucleotides by using a mass spectrometer with a sonic spray ionization source". Anal Sci 17 (10): 1179–1182. 2001. doi:10.2116/analsci.17.1179. PMID 11990592.
- ↑ "Multi-charged oligonucleotide ion formation in sonic spray ionization". Anal Sci 18 (4): 385–390. 2002. doi:10.2116/analsci.18.385. PMID 11999509.
- ↑ "Desorption sonic spray ionization for (high) voltage-free ambient mass spectrometry". Rapid Commun. Mass Spectrom. 20 (19): 2901–2905. 2006. doi:10.1002/rcm.2680. PMID 16941547. Bibcode: 2006RCMS...20.2901H.
- ↑ "Easy Ambient Sonic-Spray Ionization Mass Spectrometry Combined with Thin-Layer Chromatography". Anal. Chem. 80 (8): 2744–2750. 2008. doi:10.1021/ac702216q. PMID 18331004.
- ↑ Chen, Tsung-Yi; Lin, Jia-Yi; Chen, Jen-Yi; Chen, Yu-Chie (2010). "Ultrasonication-assisted spray ionization mass spectrometry for the analysis of biomolecules in solution". Journal of the American Society for Mass Spectrometry 21 (9): 1547–1553. doi:10.1016/j.jasms.2010.04.021. PMID 20547459.
- ↑ Chen, Tsung-Yi; Chao, Chin-Sheng; Mong, Kwok-Kong Tony; Chen, Yu-Chie (4 November 2010). "Ultrasonication-assisted spray ionization mass spectrometry for on-line monitoring of organic reactions". Chemical Communications 46 (44): 8347–9. doi:10.1039/C0CC02629H. PMID 20957254. https://zenodo.org/record/999715. Retrieved 4 November 2011.
- ↑ Alton, G. D. (1988). "Characterization of a cesium surface ionization source with a porous tungsten ionizer. I". Review of Scientific Instruments 59 (7): 1039. doi:10.1063/1.1139776. ISSN 0034-6748. Bibcode: 1988RScI...59.1039A. https://zenodo.org/record/1231832.
- ↑ "A Negative-Surface Ionization for Generation of Halogen Radioactive Ion Beams". http://www.ornl.gov/~webworks/cpr/v823/pres/110496.pdf.
- ↑ Cooks, R. Graham; Ouyang, Zheng; Takats, Zoltan; Wiseman, Justin M. (2006). "Ambient Mass Spectrometry". Science 311 (5767): 1566–70. doi:10.1126/science.1119426. PMID 16543450. Bibcode: 2006Sci...311.1566C.
- ↑ 74.0 74.1 Monge, María Eugenia; Harris, Glenn A.; Dwivedi, Prabha; Fernández, Facundo M. (2013). "Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization". Chemical Reviews 113 (4): 2269–2308. doi:10.1021/cr300309q. ISSN 0009-2665. PMID 23301684.
- ↑ Huang, Min-Zong; Yuan, Cheng-Hui; Cheng, Sy-Chyi; Cho, Yi-Tzu; Shiea, Jentaie (2010). "Ambient Ionization Mass Spectrometry". Annual Review of Analytical Chemistry 3 (1): 43–65. doi:10.1146/annurev.anchem.111808.073702. ISSN 1936-1327. PMID 20636033. Bibcode: 2010ARAC....3...43H.
- ↑ Badu-Tawiah, Abraham K.; Eberlin, Livia S.; Ouyang, Zheng; Cooks, R. Graham (2013). "Chemical Aspects of the Extractive Methods of Ambient Ionization Mass Spectrometry". Annual Review of Physical Chemistry 64 (1): 481–505. doi:10.1146/annurev-physchem-040412-110026. ISSN 0066-426X. PMID 23331308. Bibcode: 2013ARPC...64..481B.
- ↑ "Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology". Journal of Mass Spectrometry 40 (10): 1261–75. 2005. doi:10.1002/jms.922. PMID 16237663. Bibcode: 2005JMSp...40.1261T.
- ↑ "Analysis of Solids, Liquids, and Biological Tissues Using Solids Probe Introduction at Atmospheric Pressure on Commercial LC/MS Instruments". Anal. Chem. 77 (23): 7826–7831. 2005. doi:10.1021/ac051470k. PMID 16316194.
- ↑ "Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids". Rapid Commun. Mass Spectrom. 19 (24): 3701–4. 2005. doi:10.1002/rcm.2243. PMID 16299699. Bibcode: 2005RCMS...19.3701S.
- ↑ Peng, Ivory X.; Ogorzalek Loo, Rachel R.; Margalith, Eli; Little, Mark W.; Loo, Joseph A. (2010). "Electrospray-assisted laser desorption ionization mass spectrometry (ELDI-MS) with an infrared laser for characterizing peptides and proteins". The Analyst 135 (4): 767–72. doi:10.1039/b923303b. ISSN 0003-2654. PMID 20349541. Bibcode: 2010Ana...135..767P.
- ↑ Sampson, JS; Hawkridge, AM; Muddiman, DC (2006). "Generation and detection of multiply charged peptides and proteins by matrix-assisted laser desorption electrospray ionization (MALDESI) Fourier transform ion cyclotron resonance mass spectrometry". J. Am. Soc. Mass Spectrom. 17 (12): 1712–6. doi:10.1016/j.jasms.2006.08.003. PMID 16952462.
- ↑ 35 years of H- ions at Fermilab. Fermilab. pp. 12. http://www-ad.fnal.gov/proton/PIP/Communicate/Calendar/Repository/2014/35%20years%20of%20H-%20ions%20at%20Fermilab.pdf. Retrieved 12 August 2015.
- ↑ Cooks, R. G; Ouyang, Z; Takats, Z; Wiseman, J. M (2006). "Ion Beam Sources". Science 311 (5767): 1566–70. doi:10.1126/science.1119426. PMID 16543450. Bibcode: 2006Sci...311.1566C. http://www.advanced-energy.com/en/upload/File/Sources/SL-ION-230-02.pdf. Retrieved 2006-12-14.
- ↑ "Ion Beam Source Technology". Advanced Energy. http://www.advanced-energy.com/en/Ion.html.
 |