Earth:Carboniferous
| Carboniferous | |
|---|---|
| 358.9 ± 0.4 – 298.9 ± 0.15 Ma | |
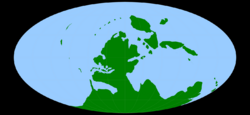 A map of the world as it appeared during the middle Carboniferous, c. 330 Ma | |
| Chronology | |
| Etymology | |
| Name formality | Formal |
| Nickname(s) | Age of Amphibians |
| Usage information | |
| Celestial body | Earth |
| Regional usage | Global (ICS) |
| Time scale(s) used | ICS Time Scale |
| Definition | |
| Chronological unit | Period |
| Stratigraphic unit | System |
| First proposed by | William Daniel Conybeare and William Phillips, 1822 |
| Time span formality | Formal |
| Lower boundary definition | FAD of the Conodont Siphonodella sulcata (discovered to have biostratigraphic issues as of 2006)[1] |
| Lower boundary GSSP | La Serre, Montagne Noire, France [ ⚑ ] 43°33′20″N 3°21′26″E / 43.5555°N 3.3573°E |
| GSSP ratified | 1990[2] |
| Upper boundary definition | FAD of the Conodont Streptognathodus isolatus within the morphotype Streptognathodus wabaunsensis chronocline |
| Upper boundary GSSP | Aidaralash, Ural Mountains, Kazakhstan [ ⚑ ] 50°14′45″N 57°53′29″E / 50.2458°N 57.8914°E |
| GSSP ratified | 1996[3] |
| Atmospheric and climatic data | |
| Sea level above present day | Falling from 120 m to present-day level throughout the Mississippian, then rising steadily to about 80 m at end of period[4] |
The Carboniferous (/ˌkɑːrbəˈnɪfərəs/ KAR-bə-NIF-ər-əs)[5] is a geologic period and system of the Paleozoic that spans 60 million years from the end of the Devonian Period 358.9 Ma (million years ago), to the beginning of the Permian Period, 298.9 Ma. In North America, the Carboniferous is often treated as two separate geological periods, the earlier Mississippian and the later Pennsylvanian.[6]
The name Carboniferous means "coal-bearing", from the Latin carbō ("coal") and ferō ("bear, carry"), and refers to the many coal beds formed globally during that time.[7] The first of the modern "system" names, it was coined by geologists William Conybeare and William Phillips in 1822,[8] based on a study of the British rock succession.
Carboniferous is the period during which both terrestrial animal and land plant life was well established.[9] Stegocephalia (four-limbed vertebrates including true tetrapods), whose forerunners (tetrapodomorphs) had evolved from lobe-finned fish during the preceding Devonian period, became pentadactylous during the Carboniferous.[10] The period is sometimes called the Age of Amphibians[11] due to the diversification of early amphibians such as the temnospondyls, which became dominant land vertebrates,[12] as well as the first appearance of amniotes including synapsids (the clade to which modern mammals belong) and sauropsids (which include modern reptiles and birds) during the late Carboniferous. Due to the raised atmospheric oxygen level, land arthropods such as arachnids (e.g. trigonotarbids and Pulmonoscorpius), myriapods (e.g. Arthropleura) and insects (e.g. Meganeura) also underwent a major evolutionary radiation during the late Carboniferous. Vast swaths of forests and swamps covered the land, which eventually became the coal beds characteristic of the Carboniferous stratigraphy evident today.
The later half of the period experienced glaciations, low sea level, and mountain building as the continents collided to form Pangaea. A minor marine and terrestrial extinction event, the Carboniferous rainforest collapse, occurred at the end of the period, caused by climate change.[13]
Etymology and history
The development of a Carboniferous chronostratigraphic timescale began in the late 18th century. The term "Carboniferous" was first used as an adjective by Irish geologist Richard Kirwan in 1799, and later used in a heading entitled "Coal-measures or Carboniferous Strata" by John Farey Sr. in 1811. Four units were originally ascribed to the Carboniferous, in ascending order, the Old Red Sandstone, Carboniferous Limestone, Millstone Grit and the Coal Measures. These four units were placed into a formalised Carboniferous unit by William Conybeare and William Phillips in 1822, and then into the Carboniferous System by Phillips in 1835. The Old Red Sandstone was later considered Devonian in age.[14]
The similarity in successions between the British Isles and Western Europe led to the development of a common European timescale with the Carboniferous System divided into the lower Dinantian, dominated by carbonate deposition and the upper Silesian with mainly siliciclastic deposition.[15] The Dinantian was divided into the Tournaisian and Viséan stages. The Silesian into the Namurian, Westphalian and Stephanian stages. The Tournaisian is the same length as the International Commission on Stratigraphy (ICS) stage, but the Viséan is longer, extending into the lower Serpukhovian.[14] North American geologists recognised a similar stratigraphy, but divided it into two systems rather than one. These are the lower carbonate-rich sequence of the Mississippian System and the upper siliciclastic and coal-rich sequence of the Pennsylvanian. The United States Geological Survey officially recognised these two systems in 1953.[16] In Russia, in the 1840s British and Russian geologists divided the Carboniferous into the Lower, Middle and Upper series based on Russian sequences. In the 1890s these became the Dinantian, Moscovian and Uralian stages. The Serpukivian was proposed as part of the Lower Carboniferous, and the Upper Carboniferous was divided into the Moscovian and Gzhelian. The Bashkirian was added in 1934.[14]
In 1975, the ICS formally ratified the Carboniferous System, with the Mississippian and Pennsylvanian subsystems from the North American timescale, the Tournaisian and Visean stages from the Western European and the Serpukhovian, Bashkirian, Moscovian, Kasimovian and Gzhelian from the Russian.[14] With the formal ratification of the Carboniferous System, the Dinantian, Silesian, Namurian, Westphalian and Stephanian became redundant terms, although the latter three are still in common use in Western Europe.[15]
Geology
Stratigraphy
The Carboniferous is divided into two subsystems; the Mississippian and Pennsylvanian. These are divided into three series and seven stages. The Tournaisian, Visean and Serpukhovian stages equate to the Lower, Middle and Upper series of the Mississippian respectively. The Bashkirian and Moscovian stages, the Lower and Middle Pennsylvanian and the Kasimovian and Gzhelian stages the Upper Pennsylvanian.[14]
Stages can be defined globally or regionally. For global stratigraphic correlation, the ICS ratify global stages based on a Global Boundary Stratotype Section and Point (GSSP) from a single formation (a stratotype) identifying the lower boundary of the stage. Only the boundaries of the Carboniferous System and three of the stage bases are defined by global stratotype sections and points because of the complexity of the geology.[17][14] The ICS subdivisions from youngest to oldest are as follows:[18]
| Series/epoch | Stage/age | Lower boundary | |
| Permian | Asselian | 298.9 ±0.15 Ma | |
| Pennsylvanian | Upper | Gzhelian | 303.7 ±0.1 Ma |
| Kasimovian | 307.0 ±0.1 Ma | ||
| Middle | Moscovian | 315.2 ±0.2 Ma | |
| Lower | Bashkirian | 323.2 ±0.4 Ma | |
| Mississippian | Upper | Serpukhovian | 330.9 ±0.2 Ma |
| Middle | Visean | 346.7 ±0.4 Ma | |
| Lower | Tournaisian | 358.9 ±0.4 Ma | |
Mississippian
The Mississippian was proposed by Alexander Winchell in 1870 named after the extensive exposure of Lower Carboniferous limestone in the upper Mississippi valley.[16] During the Mississippian, there was a marine connection between the Paleo-Tethys and Panthalassa through the Rheic Ocean resulting in the near worldwide distribution of marine faunas and so allowing widespread correlations using marine biostratigraphy.[17][14] However, there are few Mississippian volcanic rocks and so obtaining radiometric dates is difficult.[17]
The Tournaisian Stage is named after the Belgian city of Tournai. It was introduced in scientific literature by Belgian geologist André Dumont in 1832. The GSSP for the base of the Carboniferous System, Mississippian Subsystem and Tournaisian Stage is located at the La Serre section in Montagne Noire, southern France. It is defined by the first appearance of the conodont Siphonodella sulcata within the evolutionary lineage from Siphonodella praesulcata to Siphonodella sulcata. This was ratified by the ICS in 1990. However, in 2006 further study revealed the presence of Siphonodella sulcata below the boundary, and the presence of Siphonodella praesulcata and Siphonodella sulcata together above a local unconformity. This means the evolution of one species to the other, the definition of the boundary, is not seen at the La Serre site making precise correlation difficult.[14][19]
The Viséan Stage was introduced by André Dumont in 1832 and is named after the city of Visé, Liège Province, Belgium. In 1967, the base of the Visean was officially defined as the first black limestone in the Leffe facies at the Bastion Section in the Dinant Basin. These changes are now thought to be ecologically driven rather than due to evolutionary change, and so this has not been used as the location for the GSSP. Instead, the GSSP for the base of the Visean is located in Bed 83 of the sequence of dark grey limestones and shales at the Pengchong section, Guangxi, southern China. It is defined by the first appearance of the fusulinid Eoparastaffella simplex in the evolutionary lineage Eoparastaffella ovalis – Eoparastaffella simplex and was ratified in 2009.[14]
The Serpukhovian Stage was proposed in 1890 by Russian stratigrapher Sergei Nikitin. It is named after the city of Serpukhov, near Moscow. The Serpukhovian Stage currently lacks a defined GSSP. The Visean-Serpukhovian boundary coincides with a major period of glaciation. The resulting sea level fall and climatic changes led to the loss of connections between marine basins and endemism of marine fauna across the Russian margin. This means changes in biota are environmental rather than evolutionary making wider correlation difficult.[14] Work is underway in the Urals and Nashui, Guizhou Province, southwestern China for a suitable site for the GSSP with the proposed definition for the base of the Serpukhovian as the first appearance of conodont Lochriea ziegleri.[19]
Pennsylvanian
The Pennsylvanian was proposed by J.J.Stevenson in 1888, named after the widespread coal-rich strata found across the state of Pennsylvania.[16] The closure of the Rheic Ocean and formation of Pangea during the Pennsylvanian, together with widespread glaciation across Gondwana led to major climate and sea level changes, which restricted marine fauna to particular geographic areas thereby reducing widespread biostratigraphic correlations.[17][14] Extensive volcanic events associated with the assembling of Pangea means more radiometric dating is possible relative to the Mississippian.[17]
The Bashkirian Stage was proposed by Russian stratigrapher Sofia Semikhatova in 1934. It was named after Bashkiria, the then Russian name of the republic of Bashkortostan in the southern Ural Mountains of Russia. The GSSP for the base of the Pennsylvanian Subsystem and Bashkirian Stage is located at Arrow Canyon in Nevada, US and was ratified in 1996. It is defined by the first appearance of the conodont Declinognathodus noduliferus. Arrow Canyon lay in a shallow, tropical seaway which stretched from Southern California to Alaska. The boundary is within a cyclothem sequence of transgressive limestones and fine sandstones, and regressive mudstones and brecciated limestones.[14]
The Moscovian Stage is named after shallow marine limestones and colourful clays found around Moscow, Russia. It was first introduced by Sergei Nikitin in 1890. The Moscovian currently lacks a defined GSSP. The fusulinid Aljutovella aljutovica can be used to define the base of the Moscovian across the northern and eastern margins of Pangea, however, it is restricted in geographic area, which means it cannot be used for global correlations.[14] The first appearance of the conodonts Declinognathodus donetzianus or Idiognathoides postsulcatus have been proposed as a boundary marking species and potential sites in the Urals and Nashui, Guizhou Province, southwestern China are being considered.[19]
The Kasimovian is the first stage in the Upper Pennsylvanian. It is named after the Russian city of Kasimov, and was originally included as part of Nikitin's 1890 definition of the Moscovian. It was first recognised as a distinct unit by A.P. Ivanov in 1926, who named it the "Tiguliferina" Horizon after a type of brachiopod. The boundary covers of period of globally low sea level, which has resulted in disconformities within many sequences of this age. This has created difficulties in finding suitable marine fauna that can used to correlate boundaries worldwide.[14] The Kasimovian currently lacks a defined GSSP and potential sites in the southern Urals, southwest USA and Nashui, Guizhou Province, southwestern China are being considered.[19]
The Gzhelian Stage is the second stage in the Upper Pennsylvanian. It is named after the Russian village of Gzhel, near Ramenskoye, not far from Moscow. The name and type locality were defined by Sergei Nikitin in 1890. The restricted geographic distribution of fauna is again a problem in defining the Kasimovian-Gzhelian boundary and the base of the Gzhelian currently lacks a defined GSSP. The first appearance of the fusulinid Rauserites rossicus and Rauserites stuckenbergi can be used in the Boreal Sea and Paleo-Tethyan regions but not eastern Pangea or Panthalassa margins.[14] Potential sites in the Urals and Nashui, Guizhou Province, southwestern China for the GSSP are being considered.[19]
The GSSP for the base of the Permian is located in the Aidaralash River valley near Aqtöbe, Kazakhstan and was ratified in 1996. The beginning of the stage is defined by the first appearance of the conodont Streptognathodus postfusus.[20]
Cyclothems
A cyclothem is a succession of non-marine and marine sedimentary rocks, deposited during a single sedimentary cycle, with an erosional surface at its base. Whilst individual cyclothems are often only metres to a few tens of metres thick, cyclothem sequences can be many hundreds to thousands of metres thick, and contain tens to hundreds of individual cyclothems.[21] Cyclothems were deposited along continental shelves where the very gentle gradient of the shelves meant even small changes in sea level led to large advances or retreats of the sea.[16] Cyclothem lithologies vary from mudrock and carbonate-dominated to coarse siliciclastic sediment-dominated sequences depending on the paleo-topography, climate and supply of sediments to the shelf.[22]

The main period of cyclothem deposition occurred during the Late Paleozoic Ice Age (LPIA) from the Late Mississippian to Early Permian, when the waxing and waning of ice sheets led to rapid changes in eustatic sea level.[22] The growth of ice sheets led global sea levels to fall as water was lock away in glaciers. Falling sea levels exposed large tracts of the continental shelves across which river systems eroded channels and valleys and vegetation broke down the surface to form soils. The non-marine sediments deposited on this erosional surface form the base of the cyclothem.[22] As sea levels began to rise, the rivers flowed through increasingly water-logged landscapes of swamps and lakes. Peat mires developed in these wet and oxygen-poor conditions, leading to coal formation.[15] With continuing sea level rise, coastlines migrated landward and deltas, lagoons and esturaries developed; their sediments deposited over the peat mires. As fully marine conditions were established, limestones succeeded these marginal marine deposits. The limestones were in turn overlain by deep water black shales as maximum sea levels were reached.[16] Ideally, this sequence would be reversed as sea levels began to fall again, however, sea level falls tend to be protracted, whilst sea level rises are rapid - ice sheets grow slowly, but melt quickly. Therefore, the majority of a cyclothem sequence occurred during falling sea levels, when rates of erosion were high, meaning they were often periods of non-deposition. Erosion during sea level falls could also result in the full or partial removal of previous cyclothem sequences. Individual cyclothems are generally less than 10 m thick because the speed at which sea level rose gave only limited time for sediments to accumulate.[22]
During the Pennsylvanian, cyclothems were deposited in shallow, epicontinental seas across the tropical regions of Laurussia (present day western and central US, Europe, Russia and central Asia) and the North and South China cratons.[16] The rapid sea levels fluctuations they represent correlate with the glacial cycles of the Late Paleozoic Ice Age. The advance and retreat of ice sheets across Gondwana followed a 100 kyr Milankovitch cycle and so each cyclothem represents a cycle of sea level fall and rise over a 100 kyr period.[22]
Coal formation

Coal forms when organic matter builds up in waterlogged, anoxic swamps, known as peat mires, and is then buried, compressing the peat into coal. The majority of Earth's coal deposits were formed during the Late Carboniferous and Early Permian. The plants from which they formed contributed to changes in the Carboniferous Earth's atmosphere, and the coal itself fuelled the modern Industrial Revolution.[23]
During the Pennsylvanian, vast amounts of organic debris accumulated in the peat mires that formed across the low-lying, humid equatorial wetlands of the foreland basins of the Variscan-Alleghanian-Ouachita mountains in Laurussia, and around the margins of the North and South China continents.[23] During glacial periods, low sea levels exposed large areas of the continental shelves. Major river channels, up to several kilometres wide, stretched across these shelves feeding a network of smaller channels, lakes and peat mires.[15] These wetlands were then buried by sediment as sea-levels rose during interglacials. Continued crustal subsidence of the foreland basins and continental margins allowed this accumulation and burial of peat deposits to continue over millions of years resulting in the formation of thick and widespread coal formations.[23]
During the warm interglacials, smaller coal swamps with plants adapted to the temperate conditions, also formed on the Siberian craton and the western Australian region of Gondwana.[16]
There is ongoing debate as to why this peak in the formation of Earth's coal deposits occurred during the Carboniferous. The first theory, known as the delayed fungal evolution hypothesis, is that a delay between the development of trees with the wood fibre lignin and the subsequent evolution of lignin-degrading fungi gave a period of time where vast amounts of lignin-based organic material could accumulate. Genetic analysis of basidiomycete fungi, which have enzymes capable of breaking down lignin, supports this theory by suggesting this fungi evolved in the Permian.[24][25] However, significant Mesozoic and Cenozoic coal deposits formed after lignin-digesting fungi had become well established, and fungal degradation of lignin may have already evolved by the end of the Devonian, even if the specific enzymes used by basidiomycetes had not.[23] The second theory is that the geographical setting and climate of the Carboniferous were unique in Earth's history: the co-occurrence of the position of the continents across the humid equatorial zone, high biological productivity, and the low-lying, water-logged and slowly subsiding sedimentary basins that allowed the thick accumulation of peat were sufficient to account for the peak in coal formation.[23]
Palaeogeography
During the Carboniferous, there was an increased rate in tectonic plate movements as the supercontinent of Pangea assembled. The continents themselves formed a near circle around the opening Paleo-Tethys Ocean, with the massive Panthalassic Ocean beyond. The largest continent, Gondwana (modern day Africa, Arabia, South America, India , Madagascar , West Australia and East Antarctica), covered the south polar region. To its northwest was Laurussia (modern day North America, Greenland, Scandinavia, and much of Western Europe). These two continents slowly collided to form the core of Pangea. To the north of Laurussia lay Siberia and Amuria (central Mongolia). To the east of Siberia, Kazakhstania, North China and South China formed the northern margin of the Paleo-Tethys, with Annamia (Mainland Southeast Asia) laying to the south.[26]
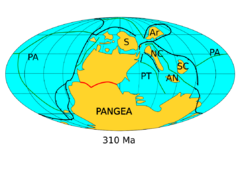
An Early Carboniferous global marine transgression resulted in the widespread deposition of limestones in the warm, shallow seas of equatorial regions. Sea levels then dropped as the Late Paleozoic Ice Age (LPIA) intensified in the Pennsylvanian, exposing large areas of continental shelf.[16] As glaciers waxed and waned repeated rises and falls in sea levels produced a distinctive pattern of terrestrial and marine sediments known as cyclothems. These consist of river channel and delta deposits with peat mires, followed by estuarine, coastal and offshore marine deposits as river deltas and wetlands built out across the continental shelves, only to be drowned as sea levels rose again.[22]
Variscan-Alleghanian-Ouachita Orogeny
Today the Variscan-Alleghanian-Ouachita Orogen stretches over 10,000 km from the present day Gulf of Mexico in the east to Turkey in the west.[27] It formed between the Middle Devonian and Early Permian as a series of continental collisions between Laurussia, Gondwana and the Armorican Terrane Assemblage (much of modern-day Central and Western Europe including Iberia) as the Rheic Ocean closed and Pangea formed.[28]
The Armorican terranes rifted away from Gondwana during the Late Ordovician. As they drifted northwards the Rheic Ocean closed in front of them and they began to collide with southeastern Laurussia in the Middle Devonian.[28] The resulting Variscan Orogeny involved a complex series of oblique collisions with associated metamorphism, igneous activity, and large-scale deformation between these terranes and Laurussia, which continued into the Carboniferous.[28]
During the mid Carboniferous, the South American sector of Gondwana collided obliquely with Laurussia's southern margin resulting in the Ouachita Orogeny.[28] The major strike-slip faulting that occurred between Laurussia and Gondwana extended eastwards into the Appalachian Mountains where early deformation in the Alleghanian Orogeny was predominantly strike-slip. As the West African sector of Gondwana collided with Laurussia, during the Late Pennsylvanian, deformation along the Alleghanian orogen became northwesterly-directed compression.[26][27]
Uralian Orogeny
The Ural Orogen is a north–south trending fold and thrust belt that forms the western edge of the Central Asian Orogenic Belt.[29] The Uralian Orogeny began in the Late Devonian and continued, with some hiatuses, into the Jurassic. From the Late Devonian to Early Carboniferous, the Magnitogorsk island arc, which lay between Kazakhstania and Laurussia in the Palaeo-Uralian Ocean, collided with the passive margin of northeastern Laurussia (Baltica craton). The suture zone between the former island arc complex and the continental margin formed the Main Uralian Fault, a major structure that runs for more than 2000 km along the orogen.(6) Accretion of the island arc was complete by the Tournaisian, but subduction of the Paleo-Ural Ocean between Kazakhstania and Laurussia continued until the Bashkirian when the ocean finally closed and continental collision began.[29] Significant strike-slip movement along this zone indicates the collision was oblique. Deformation continued into the Permian and during the Late Carboniferous and Permian the region was extensively intruded by granites.[28][29]
Laurussia
The Laurussian continent was formed by the collision between Laurentia, Baltica and Avalonia during the Devonian. At the beginning of the Carboniferous it lay at low latitude in the southern hemisphere and drifted north during the Carboniferous, crossing the equator during the mid-to-Late Carboniferous and reaching low latitudes in the northern hemisphere by the end of the Carboniferous.[26] The Variscan-Appalachian-Ouachita mountain ranges drew in moist air from the Paleo-Tethys resulting in heavy precipitation and a tropical wetland environment. Extensive coaldeposits developed within the cyclothem sequences that dominated the Pennsylvanian sedimentary basins associated with the growing orogenic belt.[16][30]
Whilst the southern and southeastern margins of Laurussia were dominated by the Variscan-Alleghanian-Ouachita Orogeny and the northeasterly margin by the Uralian Orogeny, subduction of the Panthalassic oceanic plate along its western margin resulted in the Antler Orogeny in the Late Devonian to early Mississippian. Further north along the margin, slab roll-back, beginning in the early Mississippian, led to the rifting of the Yukon-Tanana terrane and the opening of the Slide Mountain Ocean. Along the northern margin of Laurussia, orogenic collapse of the Late Devonian to early Mississippian Ellesmerian or Innuitian Orogeny led to the development of the Sverdrup Basin.[28]
Gondwana
Much of Gondwana lay in the southern polar region during the Carboniferous. As the plate moved, the South Pole drifted from southern Africa in the Early Carboniferous to East Antarctica by the end of the period.[26] Glacial deposits are widespread across Gondwana and indicate multiple ice centres and long-distance movement of ice.[21]
The northern to northeastern margin of Gondwana (Northeast Africa, Arabia, India and northeastern West Australia) was a passive margin along the southern edge of the Paleo-Tethys with cyclothem deposition including, during more temperate intervals, coal swamps in Western Australia.[26] The Mexican terranes along the northwestern Gondwanan margin, were affected by the subduction of the Rheic Ocean.[28] However, they lay to west of the Ouachita Orogeny and were not impacted by continental collision, but became part of the active margin of the Pacific.[27] The Moroccan margin was affected by periods of widespread dextral strike-slip deformation, magmatism and metamorphism associated with the Variscan Orogeny.[26]
Towards the end of the Carboniferous, extension and rifting across the northern margin of Gondwana would led to the breaking away of the Cimmerian Terrane (parts of present-day Turkey, Iran, Afghanistan, Pakistan , Tibet, China , Myanmar, Thailand and Malaysia) during the early Permian and the opening of the Neo-Tethys Ocean.[28]
Along the southeastern and southern margin of Gondwana (eastern Australia and Antarctica), northward subduction of Panthalassa continued. Changes in the relative motion of the plates resulted in the Early Carboniferous Kanimblan Orogeny. Continental arc magmatism continued into the Late Carboniferous and extended round to connect with the developing proto-Andean subduction zone along the western South American margin of Gondwana.[26]
Siberia and Amuria
Shallow seas covered much of the Siberian craton in the Early Carboniferous. These retreated as sea levels fell in the Pennsylvanian and as the continent drifted north into more temperate zones extensive coal deposits formed in the Kuznetsk Basin.[30]
The northwest to eastern margins of Siberia were passive margins along the Mongol-Okhotsk Ocean on the far side of which lay Amuria. From the mid Carboniferous, subduction zones with associated magmatic arcs developed along both margins of the ocean.[28]
The southwestern margin of Siberia was the site of the long lasting and complex accretionary orogen. The Devonian to Early Carboniferous Siberian and South Chinese Altai accretionary complexes developed above an east-dipping subduction zone, whilst further south, the Zharma-Saur arc formed along the northeastern margin of Kazakhstania.[31] By the Late Carboniferous, all these complexes had accreted to the Siberian craton as shown by the intrusion of post-orogenic granites across the region. As Kazakhstania had already accreted to Laurussia, Siberia was effectively part of Pangea by 310Ma, although major transcurrent movements continued between it and Laurussia into the Permian.[28]
Central and East Asia
The Kazakhstanian microcontinent is composed of a series of Devonian and older accretionary complexes. It was strongly deformed during the Carboniferous as its western margin collided with Laurussia during the Uralian Orogen and its northeastern margin collided with Siberia. Continuing transcurrent motion between Laurussia and Siberia led the formerly elongate microcontinent to bend into an orocline.[28]
During the Carboniferous, the Tarim craton lay along the northwestern edge of North China. Subduction along the Kazakhstanian margin of the Turkestan Ocean resulted in collision between northern Tarim and Kazakhstania during the mid Carboniferous as the ocean closed. The South Tian Shan fold and thrust belt, which extends over 2000 km from Uzbekistan to Northwest China, is the remains of this accretionary complex and forms the suture between Kazakhstania and Tarim.[28][32] A continental magmatic arc above a south-dipping subduction zone lay along the northern North China margin, consuming the Paleoasian Ocean.[26] Northward subduction of the Paleo-Tethys beneath the southern margins of North China and Tarim continued during the Carboniferous, with the South Qinling block accreted to North China during the mid to Late Carboniferous.[28]
No sediments are preserved from the Early Carboniferous in North China. However, bauxite deposits immediately above the regional mid Carboniferous unconformity indicate warm tropical conditions and are overlain by cyclothems including extensive coals.[26]
South China and Annamia (Mainland Southeast Asia) rifted from Gondwana during the Devonian.[28] During the Carboniferous, they were separated from each other and North China by the Paleoasian Ocean with the Paleo-Tethys to the southwest and Panthalassa to the northeast. Cyclothem sediments with coal and evaporites were deposited across the passive margins that surrounded both continents. Offshore eastern South China the proto-Japanese islands lay above a subduction zone consuming the Panthalassic Ocean.[26]
Climate

Average global temperatures in the Early Carboniferous Period were high: approximately 20 °C (68 °F). However, cooling during the Middle Carboniferous reduced average global temperatures to about 12 °C (54 °F). Atmospheric carbon dioxide levels fell during the Carboniferous Period from roughly 8 times the current level in the beginning, to a level similar to today's at the end.[33] The Carboniferous is considered part of the Late Palaeozoic Ice Age, which began in the latest Devonian with the formation of small glaciers in Gondwana.[34] During the Tournaisian the climate warmed, before cooling, there was another warm interval during the Viséan, but cooling began again during the early Serpukhovian. At the beginning of the Pennsylvanian around 323 million years ago, glaciers began to form around the South Pole, which grew to cover a vast area of Gondwana. This area extended from the southern reaches of the Amazon basin and covered large areas of southern Africa, as well as most of Australia and Antarctica. Cyclothems, which began around 313 million years ago, and continue into the following Permian indicate that the size of the glaciers were controlled by Milankovitch cycles akin to recent ice ages, with glacial periods and interglacials. Deep ocean temperatures during this time were cold due to the influx of cold bottom waters generated by seasonal melting of the ice cap.[35]
Although it is often asserted that Carboniferous atmospheric oxygen concentrations were significantly higher than today, at around 30% of total atmospheric concentration, prehistoric atmospheric oxygen concentration estimates are highly uncertain, with other estimates suggesting that the amount of oxygen was actually lower than that present in today's atmosphere.[36]
The cooling and drying of the climate led to the Carboniferous Rainforest Collapse (CRC) during the late Carboniferous. Tropical rainforests fragmented and then were eventually devastated by climate change.[13]
Geochemistry
As the continents assembled to form Pangea, the growth of the Variscan-Alleghanian-Ouachita mountains led to increased weathering and carbonate sedimentation on the ocean floor,[37] whilst the distribution of continents across the paleo-tropics meant vast areas of land were available for the spread of tropical rainforests.[16] Together these two factors significantly increased CO2 drawdown from the atmosphere, lowering global temperatures, increasing ocean pH and triggering the Late Paleozoic Ice Age.[37] The growth of the supercontinent also changed seafloor spreading rates and led to a decrease in the length and volume of mid-ocean ridge systems.[16]
Magnesium/calcium isotope ratios in seawater
During the Early Carboniferous, the Mg2+/Ca2+ ratio in seawater began to rise and by the mid-Mississippian aragonite seas had replaced calcite seas.[16] The concentration of calcium in seawater is largely controlled by ocean pH, and as this increased the calcium concentration was reduced. At the same time, the increase in weathering, increased the amount of magnesium entering the marine environment. As magnesium is removed from seawater and calcium added along mid-ocean ridges where seawater reacts with the newly formed lithosphere, the reduction in length of mid-ocean ridge systems increased the Mg2+/Ca2+ ratio further.[16] The Mg2+/Ca2+ ratio of the seas also affects the ability of organisms to biomineralize. The Carboniferous aragonite seas favoured those that secreted aragonite and the dominant reef builders of the time were aragonitic sponges and corals.[16]
Strontium isotopic composition of seawater
The strontium isotopic composition (87Sr/86Sr) of seawater represents a mix of strontium derived from continental weathering which is rich in 87Sr and from mantle sources e.g. mid-ocean ridges, which are relatively depleted in 87Sr. 87Sr/86Sr ratios above 0.7075 indicate continental weathering is the main source of 87Sr, whilst ratios below indicate mantle-derived sources are the principal contributor.[15]
87Sr/86Sr values varied through the Carboniferous, although they remained above 0.775, indicating continental weathering dominated as the source of 87Sr throughout. The 87Sr/86Sr during the Tournaisian was c. 0.70840, it decreased through the Visean to 0.70771 before increasing during the Serpukhovian to the lowermost Gzhelian where it plateaued at 0.70827, before decreasing again to 0.70814 at the Carboniferous-Permian boundary.[38] These variations reflect the changing influence of weathering and sediment supply to the oceans of the growing Variscan-Alleghanian-Ouachita mountain belt. By the Serpukhovian basement rocks, such as granite, had been uplifted and exposed to weathering. The decline towards the end of the Carboniferous is interpreted as a decrease in continental weathering due to the more arid conditions.[39]
Oxygen (δ18O) and carbon (δ13C) isotope ratios in seawater
Unlike Mg2+/Ca2+ and 87Sr/86Sr isotope ratios, which are consistent across the world's oceans at any one time, δ18O and δ13C preserved in the fossil record can be affected by regional factors.[38] Carboniferous δ18O and δ13C records show regional differences between the South China open-water setting and the epicontinental seas of Laurussia. These differences are due to variations in seawater salinity and evaporation between epicontinental seas relative to the more open waters.[38] However, large scale trends can still be determined. δ13C rose rapidly from c. 0 to 1‰ (parts per thousand) to c. 5 to 7‰ in the earliest Mississippian and remained high for the duration of the Late Paleozoic Ice Age (c. 3–6‰) into the earliest Permian.[38] Similarly from the Early Mississippian there was a long-term increase in δ18O values as the climate cooled.[21]
Both δ13C and δ18O records show significant global isotope changes (known as excursions) during the Carboniferous.[38] The mid-Tournaisian positive δ13C and δ18O excursions lasted between 6 and 10 million years and were also accompanied by c. 6‰ positive excursion in organic matter δ15N values,[21] a negative excursion in carbonate δ238U and a positive excursion in carbonate-associated sulphate δ34S.[38] These changes in seawater geochemistry are interpreted as a decrease in atmospheric CO2 due to increased organic matter burial and widespread ocean anoxia triggering climate cooling and onset of glaciation.[38]
The Mississippian-Pennsylvanian boundary positive δ18O excursion occurred at the same time as global sea-level falls and widespread glacial deposits across southern Gondwana, indicating climate cooling and ice build-up. The rise in 87Sr/86Sr just before the δ18O excursion suggests climate cooling in this case was due to increased continental weathering of the growing Variscan-Alleghanian-Ouachita mountains and the influence of the orogeny on precipitation and surface water flow rather than increased burial of organic matter. δ13C values show more regional variation and it is unclear whether there is a positive δ13C excursion or a readjustment from previous lower values.[38]
During the earliest Kasimovian there was a short (<1myr), intense glacial period, which came to a sudden end as atmospheric CO2 concentrations rapidly rose.[21] The Kasimovian saw a steady increase in arid conditions across tropical regions and a major reduction in the extent of tropical rainforests, as shown by the widespread loss of coal deposits from this time.[39] The resulting reduction in productivity and burial of organic matter led to increasing atmospheric CO2 levels, which were recorded by a negative δ13C excursion and an accompanying, but smaller decrease in δ18O values.[21]
Life
Plants
Early Carboniferous land plants, some of which were preserved in coal balls, were very similar to those of the preceding Late Devonian, but new groups also appeared at this time. The main Early Carboniferous plants were the Equisetales (horse-tails), Sphenophyllales (scrambling plants), Lycopodiales (club mosses), Lepidodendrales (scale trees), Filicales (ferns), Medullosales (informally included in the "seed ferns", an assemblage of a number of early gymnosperm groups) and the Cordaitales. These continued to dominate throughout the period, but during late Carboniferous, several other groups, Cycadophyta (cycads), the Callistophytales (another group of "seed ferns"), and the Voltziales, appeared.
The Carboniferous lycophytes of the order Lepidodendrales, which are cousins (but not ancestors) of the tiny club-moss of today, were huge trees with trunks 30 meters high and up to 1.5 meters in diameter. These included Lepidodendron (with its cone called Lepidostrobus), Anabathra, Lepidophloios and Sigillaria.[40] The roots of several of these forms are known as Stigmaria. Unlike present-day trees, their secondary growth took place in the cortex, which also provided stability, instead of the xylem.[41] The Cladoxylopsids were large trees, that were ancestors of ferns, first arising in the Carboniferous.[42]
| Wikisource has the text of the 1879 American Cyclopædia article Coal Plants. |
The fronds of some Carboniferous ferns are almost identical with those of living species. Probably many species were epiphytic. Fossil ferns and "seed ferns" include Pecopteris, Cyclopteris, Neuropteris, Alethopteris, and Sphenopteris; Megaphyton and Caulopteris were tree ferns.[40]
The Equisetales included the common giant form Calamites, with a trunk diameter of 30 to 60 cm (24 in) and a height of up to 20 m (66 ft). Sphenophyllum was a slender climbing plant with whorls of leaves, which was probably related both to the calamites and the lycopods.[40]
Cordaites, a tall plant (6 to over 30 meters) with strap-like leaves, was related to the cycads and conifers; the catkin-like reproductive organs, which bore ovules/seeds, is called Cardiocarpus. These plants were thought to live in swamps. True coniferous trees (Walchia, of the order Voltziales) appear later in the Carboniferous,[40] and preferred higher drier ground.
Marine invertebrates
In the oceans the marine invertebrate groups are the Foraminifera, corals, Bryozoa, Ostracoda, brachiopods, ammonoids, hederelloids, microconchids and echinoderms (especially crinoids).[citation needed] The diversity of brachiopods and fusilinid foraminiferans, surged beginning in the Visean, continuing through the end of the Carboniferous, although cephalopod and nektonic conodont diversity declined. This evolutionary radiation was known as the Carboniferous-Earliest Permian Biodiversification Event.[43] For the first time foraminifera take a prominent part in the marine faunas. The large spindle-shaped genus Fusulina and its relatives were abundant in what is now Russia, China, Japan, North America; other important genera include Valvulina, Endothyra, Archaediscus, and Saccammina (the latter common in Britain and Belgium). Some Carboniferous genera are still extant. The first true priapulids appeared during this period.[40]
The microscopic shells of radiolarians are found in cherts of this age in the Culm of Devon and Cornwall, and in Russia, Germany and elsewhere. Sponges are known from spicules and anchor ropes,[40] and include various forms such as the Calcispongea Cotyliscus and Girtycoelia, the demosponge Chaetetes, and the genus of unusual colonial glass sponges Titusvillia.
Both reef-building and solitary corals diversify and flourish; these include both rugose (for example, Caninia, Corwenia, Neozaphrentis), heterocorals, and tabulate (for example, Chladochonus, Michelinia) forms. Conularids were well represented by Conularia
Bryozoa are abundant in some regions; the fenestellids including Fenestella, Polypora, and Archimedes, so named because it is in the shape of an Archimedean screw. Brachiopods are also abundant;[44] they include productids, some of which reached very large for brachiopods size and had very thick shells (for example, the 30 cm (12 in)-wide Gigantoproductus[45][46]), while others like Chonetes were more conservative in form. Athyridids, spiriferids, rhynchonellids, and terebratulids are also very common. Inarticulate forms include Discina and Crania. Some species and genera had a very wide distribution with only minor variations.
Annelids such as Serpulites are common fossils in some horizons. Among the mollusca, the bivalves continue to increase in numbers and importance. Typical genera include Aviculopecten, Posidonomya, Nucula, Carbonicola, Edmondia, and Modiola. Gastropods are also numerous, including the genera Murchisonia, Euomphalus, Naticopsis.[40] Nautiloid cephalopods are represented by tightly coiled nautilids, with straight-shelled and curved-shelled forms becoming increasingly rare. Goniatite ammonoids such as Aenigmatoceras are common.
Trilobites are rarer than in previous periods, on a steady trend towards extinction, represented only by the proetid group. Ostracoda, a class of crustaceans, were abundant as representatives of the meiobenthos; genera included Amphissites, Bairdia, Beyrichiopsis, Cavellina, Coryellina, Cribroconcha, Hollinella, Kirkbya, Knoxiella, and Libumella.
Crinoids were highly numerous during the Carboniferous, though they suffered a gradual decline in diversity during the middle Mississippian.[47] Dense submarine thickets of long-stemmed crinoids appear to have flourished in shallow seas, and their remains were consolidated into thick beds of rock. Prominent genera include Cyathocrinus, Woodocrinus, and Actinocrinus. Echinoids such as Archaeocidaris and Palaeechinus were also present. The blastoids, which included the Pentreinitidae and Codasteridae and superficially resembled crinoids in the possession of long stalks attached to the seabed, attain their maximum development at this time.[40]
Syringothyris sp.; a spiriferid brachiopod from the Logan Formation (Lower Carboniferous) of Wooster, Ohio (internal mold)
Palaeophycus ichnosp.; a trace fossil from the Logan Formation (Lower Carboniferous) of Wooster, Ohio
Crinoid calyx from the Lower Carboniferous of Ohio with a conical platyceratid gastropod (Palaeocapulus acutirostre) attached
Essexella was a cnidarian that lived in Northern Illinois. It was long considered a scyphozoan, but is now regarded as a Sea anemone
Concavicaris was a long lasting genus of thylacocephalan arthropod that lived from the Devonian to the Carboniferous.
Triproetus was a genus of proetid trilobite, which were the only order that survived the end-Devonian extinction
Daidal was a basal species of Mantis shrimp (stomatopoda)
Syllipsimopodi was the earliest known vampyropod cephalopod, originating from Carboniferous rocks of Montana.
Freshwater and lagoonal invertebrates
Freshwater Carboniferous invertebrates include various bivalve molluscs that lived in brackish or fresh water, such as Anthraconaia, Naiadites, and Carbonicola; diverse crustaceans such as Candona, Carbonita, Darwinula, Estheria, Acanthocaris, Dithyrocaris, and Anthrapalaemon.
The eurypterids were also diverse, and are represented by such genera as Adelophthalmus, Megarachne (originally misinterpreted as a giant spider, hence its name) and the specialised very large Hibbertopterus. Many of these were amphibious.
Frequently a temporary return of marine conditions resulted in marine or brackish water genera such as Lingula, Orbiculoidea, and Productus being found in the thin beds known as marine bands.
Adelophthalmus was the only genus of eurypterine eurypterid that survived past the Devonian
Terrestrial invertebrates
Fossil remains of air-breathing insects,[48] myriapods and arachnids[49] are known from the Carboniferous. Their diversity when they do appear, however, shows that these arthropods were both well-developed and numerous.[50][51][52] Some arthropods grew to large sizes with the up to 2.6-meter-long (8.5 ft) millipede-like Arthropleura being the largest-known land invertebrate of all time. Among the insect groups are the huge predatory Protodonata (griffinflies), among which was Meganeura, a giant dragonfly-like insect and with a wingspan of ca. 75 cm (30 in)—the largest flying insect ever to roam the planet. Further groups are the Syntonopterodea (relatives of present-day mayflies), the abundant and often large sap-sucking Palaeodictyopteroidea, the diverse herbivorous Protorthoptera, and numerous basal Dictyoptera (ancestors of cockroaches).[48] Many insects have been obtained from the coalfields of Saarbrücken and Commentry, and from the hollow trunks of fossil trees in Nova Scotia. Some British coalfields have yielded good specimens: Archaeoptilus, from the Derbyshire coalfield, had a large wing with 4.3 cm (2 in) preserved part, and some specimens (Brodia) still exhibit traces of brilliant wing colors. In the Nova Scotian tree trunks land snails (Archaeozonites, Dendropupa) have been found.[53]
The late Carboniferous giant dragonfly-like insect Meganeura grew to wingspans of 75 cm (2 ft 6 in).
The gigantic Pulmonoscorpius from the early Carboniferous reached a length of up to 70 cm (2 ft 4 in).
Arthropleura was a giant millipede that fed on the Carboniferous plants. At 8 feet long, it was the largest terrestrial arthropod that ever lived.
Mazothairos was a large palaeodictyopteran insect from Mazon Creek.
Helenodora inopinata, a stem-group onychophoran known from Indiana
Maiocercus was a trigonotarbid arachnid that lived in the United Kingdom around 310 million years ago.
Fish
Many fish inhabited the Carboniferous seas; predominantly Elasmobranchs (sharks and their relatives). These included some, like Psammodus, with crushing pavement-like teeth adapted for grinding the shells of brachiopods, crustaceans, and other marine organisms. Other groups of elasmobranchs, like the ctenacanthiformes grew to large sizes, with some genera like Saivodus reaching around 6–9 meters (20–30 feet).[54] Other fish had piercing teeth, such as the Symmoriida; some, the petalodonts, had peculiar cycloid cutting teeth. Most of the other cartilaginous fish were marine, but others like the Xenacanthida, and several genera like Bandringa invaded fresh waters of the coal swamps.[55] Among the bony fish, the Palaeonisciformes found in coastal waters also appear to have migrated to rivers. Sarcopterygian fish were also prominent, and one group, the Rhizodonts, reached very large size.
Most species of Carboniferous marine fish have been described largely from teeth, fin spines and dermal ossicles,[40] with smaller freshwater fish preserved whole.
Freshwater fish were abundant, and include the genera Ctenodus, Uronemus, Acanthodes, Cheirodus, and Gyracanthus.
Chondrichthyes (especially holocephalans like the Stethacanthids) underwent a major evolutionary radiation during the Carboniferous.[56] It is believed that this evolutionary radiation occurred because the decline of the placoderms at the end of the Devonian Period caused many environmental niches to become unoccupied and allowed new organisms to evolve and fill these niches.[56] As a result of the evolutionary radiation Carboniferous holocephalans assumed a wide variety of bizarre shapes including Stethacanthus which possessed a flat brush-like dorsal fin with a patch of denticles on its top.[56] Stethacanthus's unusual fin may have been used in mating rituals.[56] Other groups like the eugeneodonts filled in the niches left by large predatory placoderms. These fish were unique as they only possessed one row of teeth in their upper or lower jaws in the form of elaborate tooth whorls.[57] The first members of the helicoprionidae, a family eugeneodonts that were characterized by the presence of one circular tooth whorl in the lower jaw, appeared during the lower Carboniferous.[58] Perhaps the most bizarre radiation of holocephalans at this time was that of the iniopterygiformes, an order of holocephalans that greatly resembled modern day flying fish that could have also "flown" in the water with their massive, elongated pectoral fins. They were further characterized by their large eye sockets, club-like structures on their tails, and spines on the tips of their fins.
Akmonistion of the Holocephali order Symmoriida roamed the oceans of the early Carboniferous.
Falcatus was a Carboniferous holocephalan, with a high degree of sexual dimorphism.
Dracopristis was a Ctenacanthiform elasmobranch from the late Carboniferous of New Mexico.
Ornithoprion was a small-sized Eugeneodont holocephalan that had an elongated lower jaw.
Allenypterus was a Coelacanth fish known from the Bear Gulch Limestone in Montana.
Phanerosteon was a Bony fish belonging to the extinct order Palaeonisciformes.
Edestus was a large eugeneodontid fish that possessed two tooth whorls in its mouth
Rhizodus was a large freshwater Rhizodont sarcopterygian from Europe and North America.
Squatinactis, a genus of elasmobranch fish from Montana that possessed enlarged pectoral fins similar to modern angel sharks
Bandringa is a bizarre elasmobranch fish that lived in Illinois, Ohio and Pennsylvania during the Moscovian stage. It superficially resembled a paddlefish, with an elongated upper rostrum.
Iniopteryx was a holocephalan that lived in North America. This fish belonged to a group called the Iniopterygiformes, that possibly lived like flying fish.
Listracanthus was an enigmatic chondrichthyean (potentially an elasmobranch) that possessed quill-like dermal denticles on its back
Restoration of Strigilodus, a petalodont holocephalan from the upper Carboniferous of Kentucky.
Tetrapods
Carboniferous amphibians were diverse and common by the middle of the period, more so than they are today; some were as long as 6 meters, and those fully terrestrial as adults had scaly skin.[59] They included a number of basal tetrapod groups classified in early books under the Labyrinthodontia. These had long bodies, a head covered with bony plates and generally weak or undeveloped limbs.[53] The largest were over 2 meters long. They were accompanied by an assemblage of smaller amphibians included under the Lepospondyli, often only about 15 cm (6 in) long. Some Carboniferous amphibians were aquatic and lived in rivers (Loxomma, Eogyrinus, Proterogyrinus); others may have been semi-aquatic (Ophiderpeton, Amphibamus, Hyloplesion) or terrestrial (Dendrerpeton, Tuditanus, Anthracosaurus).
The Carboniferous Rainforest Collapse slowed the evolution of amphibians who could not survive as well in the cooler, drier conditions. Amniotes, however, prospered due to specific key adaptations.[13] One of the greatest evolutionary innovations of the Carboniferous was the amniote egg, which allowed the laying of eggs in a dry environment, as well as keratinized scales and claws, allowing for the further exploitation of the land by certain tetrapods. These included the earliest sauropsid reptiles (Hylonomus), and the earliest known synapsid (Archaeothyris). Synapsids quickly became huge and diversified in the Permian, only for their dominance to stop during the Mesozoic Era. Sauropsids (reptiles, and also, later, birds) also diversified but remained small until the Mesozoic, during which they dominated the land, as well as the water and sky, only for their dominance to stop during the Cenozoic Era.
Reptiles underwent a major evolutionary radiation in response to the drier climate that preceded the rainforest collapse.[13][60] By the end of the Carboniferous Period, amniotes had already diversified into a number of groups, including several families of synapsid pelycosaurs, protorothyridids, captorhinids, saurians and araeoscelids.
The amphibian-like Pederpes, the most primitive tetrapod found in the Mississippian, and known from Scotland.
Hylonomus, the earliest sauropsid reptile, appeared in the Pennsylvanian, and is known from the Joggins Formation in Nova Scotia, and possibly New Brunswick.
Petrolacosaurus, the earliest known diapsid reptile, lived during the late Carboniferous.
Archaeothyris is the oldest known synapsid, and is found in rocks from Nova Scotia.
Coloraderpeton was a snake-like aïstopod tetrapodomorph from the late Carboniferous of Colorado.
Crassygyrinus was a carnivorous stem-tetrapod from the early Carboniferous of Scotland.
Microbrachis was a lepospondyl amphibian known from the Czech Republic.
Amphibamus was a dissorophoid temnospondyl from the Late Carboniferous of Illinois.
Fungi
As plants and animals were growing in size and abundance in this time (for example, Lepidodendron), land fungi diversified further. Marine fungi still occupied the oceans. All modern classes of fungi were present in the Late Carboniferous (Pennsylvanian Epoch).[61]
During the Carboniferous, animals and bacteria had great difficulty with processing the lignin and cellulose that made up the gigantic trees of the period. Microbes had not evolved that could process them. The trees, after they died, simply piled up on the ground, occasionally becoming part of long-running wildfires after a lightning strike, with others very slowly degrading into coal. White rot fungus were the first organisms to be able to process these and break them down in any reasonable quantity and timescale. Thus, some have proposed that fungi helped end the Carboniferous Period, stopping accumulation of undegraded plant matter,[62][63] although this idea remains highly controversial.[23]
Extinction events
Romer's gap
The first 15 million years of the Carboniferous had very limited terrestrial fossils. This gap in the fossil record is called Romer's gap after the American palaentologist Alfred Romer. While it has long been debated whether the gap is a result of fossilisation or relates to an actual event, recent work indicates the gap period saw a drop in atmospheric oxygen levels, indicating some sort of ecological collapse.[64] The gap saw the demise of the Devonian fish-like ichthyostegalian labyrinthodonts, and the rise of the more advanced temnospondyl and reptiliomorphan amphibians that so typify the Carboniferous terrestrial vertebrate fauna.
Carboniferous rainforest collapse
Before the end of the Carboniferous Period, an extinction event occurred. On land this event is referred to as the Carboniferous Rainforest Collapse (CRC).[13] Vast tropical rainforests collapsed suddenly as the climate changed from hot and humid to cool and arid. This was likely caused by intense glaciation and a drop in sea levels.[65]
The new climatic conditions were not favorable to the growth of rainforest and the animals within them. Rainforests shrank into isolated islands, surrounded by seasonally dry habitats. Towering lycopsid forests with a heterogeneous mixture of vegetation were replaced by much less diverse tree-fern dominated flora.
Amphibians, the dominant vertebrates at the time, fared poorly through this event with large losses in biodiversity; reptiles continued to diversify due to key adaptations that let them survive in the drier habitat, specifically the hard-shelled egg and scales, both of which retain water better than their amphibian counterparts.[13]
See also
- List of Carboniferous tetrapods
- Carboniferous rainforest collapse
- Important Carboniferous Lagerstätten
- Granton Shrimp Bed; 359 mya; Edinburgh, Scotland
- East Kirkton Quarry; c. 350 mya; Bathgate, Scotland
- Bear Gulch Limestone; 324 mya; Montana, US
- Mazon Creek; 309 mya; Illinois, US
- Hamilton Quarry; 300 mya; Kansas , US
- List of fossil sites (with link directory)
References
- ↑ Kaiser 2009.
- ↑ Paproth, Feist & Flajs 1991.
- ↑ Davydov et al. 1998.
- ↑ Haq & Schutter 2008.
- ↑ Wells 2008.
- ↑ University of California, Berkeley 2012.
- ↑ Cossey et al. 2004, p. 3.
- ↑ Conybeare & Phillips 1822, p. 323: "Book III. Medial or Carboniferous Order.".
- ↑ Garwood & Edgecombe 2011.
- ↑ Irisarri, I., Baurain, D., Brinkmann, H. et al. Phylotranscriptomic consolidation of the jawed vertebrate timetree. Nat Ecol Evol 1, 1370–1378 (2017). https://doi.org/10.1038/s41559-017-0240-5
- ↑ "Carboniferous Period". Encyclopædia Britannica. https://www.britannica.com/science/Carboniferous-Period/Carboniferous-life.
- ↑ "Animal Life in the Paleozoic". http://www.columbia.edu/~vjd1/pz_life.htm.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Sahney, Benton & Falcon-Lang 2010.
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 Davydov, V.I.; Korn, D.; Schmitz, M.D.; Gradstein, F.M.; Hammer, O. (2012), "The Carboniferous Period" (in en), The Geologic Time Scale (Elsevier): pp. 603–651, doi:10.1016/b978-0-444-59425-9.00023-8, ISBN 978-0-444-59425-9, https://linkinghub.elsevier.com/retrieve/pii/B9780444594259000238, retrieved 2021-06-17
- ↑ 15.0 15.1 15.2 15.3 15.4 Woodcock, Nigel H., ed (2012). Geological history of Britain and Ireland (2nd ed.). Chichester: Wiley-Blackwell. ISBN 978-1-4051-9381-8.
- ↑ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12 16.13 Stanley, Steven; Luczaj, John (2015). Earth System History (4th ed.). New York: W.H.Freeman and Company. ISBN 978-1-319-15402-8.
- ↑ 17.0 17.1 17.2 17.3 17.4 Lucas, Spencer G.; Schneider, Joerg W.; Nikolaeva, Svetlana; Wang, Xiangdong (2022). "The Carboniferous timescale: an introduction" (in en). Geological Society, London, Special Publications 512 (1): 1–17. doi:10.1144/SP512-2021-160. ISSN 0305-8719. Bibcode: 2022GSLSP.512....1L. https://www.lyellcollection.org/doi/10.1144/SP512-2021-160.
- ↑ Cohen, K.M., Finney, S.C., Gibbard, P.L. & Fan, J.-X. (2013; updated) The ICS International Chronostratigraphic Chart. Episodes 36: 199-204.
- ↑ 19.0 19.1 19.2 19.3 19.4 "International Commission on Stratigraphy". https://stratigraphy.org/gssps/visean.
- ↑ Davydov, V.I., Glenister, B.F., Spinosa, C., Ritter, S.M., Chernykh, V.V., Wardlaw, B.R. & Snyder, W.S. 1998. Proposal of Aidaralash as Global Stratotype Section and Point (GSSP) for base of the Permian System. Episodes, 21, 11–17.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 Montañez, Isabel Patricia (July 2022). "Current synthesis of the penultimate icehouse and its imprint on the Upper Devonian through Permian stratigraphic record" (in en). Geological Society, London, Special Publications 512 (1): 213–245. doi:10.1144/SP512-2021-124. ISSN 0305-8719. Bibcode: 2022GSLSP.512..213M.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Fielding, Christopher R. (2021-06-01). "Late Palaeozoic cyclothems – A review of their stratigraphy and sedimentology". Earth-Science Reviews 217: 103612. doi:10.1016/j.earscirev.2021.103612. ISSN 0012-8252. Bibcode: 2021ESRv..21703612F. https://www.sciencedirect.com/science/article/pii/S0012825221001124.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 Nelsen, Matthew C.; DiMichele, William A.; Peters, Shanan E.; Boyce, C. Kevin (19 January 2016). "Delayed fungal evolution did not cause the Paleozoic peak in coal production". Proceedings of the National Academy of Sciences of the United States of America 113 (9): 2442–2447. doi:10.1073/pnas.1517943113. PMID 26787881. Bibcode: 2016PNAS..113.2442N.
- ↑ Floudas, Dimitrios; Binder, Manfred; Riley, Robert; Barry, Kerrie; Blanchette, Robert A.; Henrissat, Bernard; Martínez, Angel T.; Otillar, Robert et al. (2012-06-01). "The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes". Science 336 (6089): 1715. doi:10.1126/science.1221748. ISSN 0036-8075. Bibcode: 2012Sci...336.1715F. https://ui.adsabs.harvard.edu/abs/2012Sci...336.1715F.
- ↑ Biello, David. "White Rot Fungi Slowed Coal Formation" (in en). https://www.scientificamerican.com/article/mushroom-evolution-breaks-down-lignin-slows-coal-formation/.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 Torsvik, Trond; Cocks, L.Robin (2017). Earth History and Palaeogeography. Cambridge: Cambridge University Press. ISBN 978-1-107-10532-4.
- ↑ 27.0 27.1 27.2 Nance, R. Damian; Gutiérrez-Alonso, Gabriel; Keppie, J. Duncan; Linnemann, Ulf; Murphy, J. Brendan; Quesada, Cecilio; Strachan, Rob A.; Woodcock, Nigel H. (March 2010). "Evolution of the Rheic Ocean" (in en). Gondwana Research 17 (2–3): 194–222. doi:10.1016/j.gr.2009.08.001. Bibcode: 2010GondR..17..194N. https://linkinghub.elsevier.com/retrieve/pii/S1342937X09001543.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 28.11 28.12 28.13 Domeier, Mathew; Torsvik, Trond H. (2014-05-01). "Plate tectonics in the late Paleozoic". Geoscience Frontiers 5 (3): 303–350. doi:10.1016/j.gsf.2014.01.002. ISSN 1674-9871. Bibcode: 2014GeoFr...5..303D.
- ↑ 29.0 29.1 29.2 Puchkov, Victor N. (January 2009). "The evolution of the Uralian orogen" (in en). Geological Society, London, Special Publications 327 (1): 161–195. doi:10.1144/SP327.9. ISSN 0305-8719. Bibcode: 2009GSLSP.327..161P. https://www.lyellcollection.org/doi/10.1144/SP327.9.
- ↑ 30.0 30.1 Kent, D.V.; Muttoni, G. (1 September 2020). "Pangea B and the Late Paleozoic Ice Age". Palaeogeography, Palaeoclimatology, Palaeoecology 553: 109753. doi:10.1016/j.palaeo.2020.109753. Bibcode: 2020PPP...55309753K. https://www.sciencedirect.com/science/article/abs/pii/S003101822030198X. Retrieved 17 September 2022.
- ↑ Xu, Yan; Han, Bao-Fu; Liao, Wen; Li, Ang (March 2022). "The Serpukhovian–Bashkirian Amalgamation of Laurussia and the Siberian Continent and Implications for Assembly of Pangea" (in en). Tectonics 41 (3). doi:10.1029/2022TC007218. ISSN 0278-7407. Bibcode: 2022Tecto..4107218X. https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2022TC007218.
- ↑ Alexeiev, Dmitriy V.; Cook, Harry E.; Djenchuraeva, Alexandra V.; Mikolaichuk, Alexander V. (January 2017). "The stratigraphic, sedimentological and structural evolution of the southern margin of the Kazakhstan continent in the Tien Shan Range during the Devonian to Permian" (in en). Geological Society, London, Special Publications 427 (1): 231–269. doi:10.1144/SP427.3. ISSN 0305-8719. Bibcode: 2017GSLSP.427..231A. https://www.lyellcollection.org/doi/10.1144/SP427.3.
- ↑ Stanley 1999.
- ↑ Montañez, Isabel Patricia (2 December 2021). "Current synthesis of the penultimate icehouse and its imprint on the Upper Devonian through Permian stratigraphic record". Geological Society, London, Special Publications 512 (1): 213–245. doi:10.1144/SP512-2021-124. Bibcode: 2022GSLSP.512..213M.
- ↑ Scotese, Christopher R.; Song, Haijun; Mills, Benjamin J.W.; van der Meer, Douwe G. (April 2021). "Phanerozoic paleotemperatures: The earth's changing climate during the last 540 million years". Earth-Science Reviews 215: 103503. doi:10.1016/j.earscirev.2021.103503. ISSN 0012-8252. Bibcode: 2021ESRv..21503503S. http://dx.doi.org/10.1016/j.earscirev.2021.103503. Alt URL
- ↑ Brand, Uwe; Davis, Alyssa M.; Shaver, Kristen K.; Blamey, Nigel J.F.; Heizler, Matt; Lécuyer, Christophe (May 2021). "Atmospheric oxygen of the Paleozoic" (in en). Earth-Science Reviews 216: 103560. doi:10.1016/j.earscirev.2021.103560. Bibcode: 2021ESRv..21603560B.
- ↑ 37.0 37.1 Turchyn, Alexandra V.; DePaolo, Donald J. (2019-05-30). "Seawater Chemistry Through Phanerozoic Time" (in en). Annual Review of Earth and Planetary Sciences 47 (1): 197–224. doi:10.1146/annurev-earth-082517-010305. ISSN 0084-6597. Bibcode: 2019AREPS..47..197T.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 38.7 Chen, Jitao; Chen, Bo; Montañez, Isabel P. (2022). "Carboniferous isotope stratigraphy" (in en). Geological Society, London, Special Publications 512 (1): 197–211. doi:10.1144/SP512-2020-72. ISSN 0305-8719. Bibcode: 2022GSLSP.512..197C. https://www.lyellcollection.org/doi/10.1144/SP512-2020-72.
- ↑ 39.0 39.1 Chen, Jitao; Montañez, Isabel P.; Qi, Yuping; Shen, Shuzhong; Wang, Xiangdong (2018-05-01). "Strontium and carbon isotopic evidence for decoupling of pCO2 from continental weathering at the apex of the late Paleozoic glaciation" (in en). Geology 46 (5): 395–398. doi:10.1130/G40093.1. ISSN 0091-7613. Bibcode: 2018Geo....46..395C.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 40.8 Howe 1911, p. 311.
- ↑ Westfälische Wilhelms-Universität Münster 2012.
- ↑ Hogan 2010.
- ↑ Shi, Yukun; Wang, Xiangdong; Fan, Junxuan; Huang, Hao; Xu, Huiqing; Zhao, Yingying; Shen, Shuzhong (September 2021). "Carboniferous-earliest Permian marine biodiversification event (CPBE) during the Late Paleozoic Ice Age". Earth-Science Reviews 220: 103699. doi:10.1016/j.earscirev.2021.103699. Bibcode: 2021ESRv..22003699S. https://www.sciencedirect.com/science/article/pii/S0012825221002002. Retrieved 24 August 2022.
- ↑ Pérez-Huerta, Alberto; Sheldon, Nathan D. (30 January 2006). "Pennsylvanian sea level cycles, nutrient availability and brachiopod paleoecology". Palaeogeography, Palaeoclimatology, Palaeoecology 230 (3–4): 264–279. doi:10.1016/j.palaeo.2005.07.020. Bibcode: 2006PPP...230..264P. https://www.sciencedirect.com/science/article/abs/pii/S0031018205004451. Retrieved 31 March 2023.
- ↑ Hall, Brian Keith; Müller, Gerd B.; Pearson, Roy Douglas (2004). Environment, Development, and Evolution. Toward a Synthesis. MIT Press. p. 87. ISBN 9780262083195. https://books.google.com/books?id=65Bdfy-SOyMC&dq=largest+Gigantoproductus&pg=PA87. Retrieved 2022-08-23.
- ↑ George R. McGhee, Jr. (2019). Convergent Evolution on Earth. Lessons for the Search for Extraterrestrial Life. MIT Press. p. 47. ISBN 9780262354189. https://books.google.com/books?id=bL60DwAAQBAJ&dq=largest+Gigantoproductus+giganteus&pg=PA47. Retrieved 2022-08-23.
- ↑ Ausich, William I.; Kammer, Thomas W.; Baumiller, Tomasz K. (8 February 2016). "Demise of the middle Paleozoic crinoid fauna: a single extinction event or rapid faunal turnover?". Paleobiology 20 (3): 345–361. doi:10.1017/S0094837300012811. https://www.cambridge.org/core/journals/paleobiology/article/abs/demise-of-the-middle-paleozoic-crinoid-fauna-a-single-extinction-event-or-rapid-faunal-turnover/CCE6603D020FBCE7D793746990C5011A. Retrieved 21 April 2023.
- ↑ 48.0 48.1 Garwood & Sutton 2010.
- ↑ Garwood, Dunlop & Sutton 2009.
- ↑ Graham, Jeffrey B.; Aguilar, Nancy M.; Dudley, Robert; Gans, Carl (11 May 1995). "Implications of the late Palaeozoic oxygen pulse for physiology and evolution". Nature 375 (6527): 117–120. doi:10.1038/375117a0. Bibcode: 1995Natur.375..117G. https://www.nature.com/articles/375117a0?error=cookies_not_supported&code=23b4f690-2f27-4c0f-928c-5245d4b0d4f6#citeas. Retrieved 6 November 2022.
- ↑ Cannell, Alan; Blamey, Nigel; Brand, Uwe; Escapa, Ignacio; Large, Ross (August 2022). "A revised sedimentary pyrite proxy for atmospheric oxygen in the Paleozoic: Evaluation for the Silurian-Devonian-Carboniferous period and the relationship of the results to the observed biosphere record". Earth-Science Reviews 231: 104062. doi:10.1016/j.earscirev.2022.104062. Bibcode: 2022ESRv..23104062C. https://www.sciencedirect.com/science/article/abs/pii/S0012825222001465. Retrieved 6 November 2022.
- ↑ Verberk & Bilton 2011.
- ↑ 53.0 53.1 Howe 1911, p. 312.
- ↑ Engelman, Russell K. (2023). "A Devonian Fish Tale: A New Method of Body Length Estimation Suggests Much Smaller Sizes for Dunkleosteus terrelli (Placodermi: Arthrodira)" (in en). Diversity 15 (3): 318. doi:10.3390/d15030318. ISSN 1424-2818.
- ↑ Sallan, Lauren Cole; Coates, Michael I. (January 2014). "The long-rostrumed elasmobranch Bandringa Zangerl, 1969, and taphonomy within a Carboniferous shark nursery" (in en). Journal of Vertebrate Paleontology 34 (1): 22–33. doi:10.1080/02724634.2013.782875. ISSN 0272-4634. Bibcode: 2014JVPal..34...22S. http://www.tandfonline.com/doi/abs/10.1080/02724634.2013.782875.
- ↑ 56.0 56.1 56.2 56.3 Martin 2008.
- ↑ Lebedev, O.A. (2009). "A new specimen of Helicoprion Karpinsky, 1899 from Kazakhstanian Cisurals and a new reconstruction of its tooth whorl position and function". Acta Zoologica 90: 171–182. doi:10.1111/j.1463-6395.2008.00353.x. ISSN 0001-7272. https://www.researchgate.net/publication/249440368.
- ↑ Cicimurri, D. J.; Fahrenbach, M. D. (2002). "Chondrichthyes from the upper part of the Minnelusa Formation (Middle Pennsylvanian: Desmoinesian), Meade County, South Dakota". Proceedings of the South Dakota Academy of Science 81: 81–92. http://www.sdaos.org/wp-content/uploads/pdfs/2002/81-92.pdf.
- ↑ Stanley 1999, pp. 411–412.
- ↑ Kazlev 1998.
- ↑ Blackwell et al. 2008.
- ↑ Krulwich 2016.
- ↑ Hibbett, David; Blanchette, Robert; Kenrick, Paul; Mills, Benjamin (11 July 2016). "Climate, decay, and the death of the coal forests". Current Biology 26 (13): R563–R567. doi:10.1016/j.cub.2016.01.014. PMID 27404250.
- ↑ Ward et al. 2006.
- ↑ Heckel 2008.
Sources
- Beerling, David (2007). The Emerald Planet: How Plants Changed Earth's History. Oxford University Press. ISBN 9780192806024.
- "The Carboniferous Period". http://www.ucmp.berkeley.edu/carboniferous/carboniferous.php.
- Biello, David (28 June 2012). "White Rot Fungi Slowed Coal Formation". Scientific American. http://www.scientificamerican.com/article.cfm?id=mushroom-evolution-breaks-down-lignin-slows-coal-formation. Retrieved 8 March 2013.
- Blackwell, Meredith; Vilgalys, Rytas; James, Timothy Y.; Taylor, John W. (2008). "Fungi. Eumycota: mushrooms, sac fungi, yeast, molds, rusts, smuts, etc". http://tolweb.org/Fungi/2377.
- Conybeare, W. D.; Phillips, William (1822). Outlines of the geology of England and Wales : with an introductory compendium of the general principles of that science, and comparative views of the structure of foreign countries. Part I.. London: William Phillips. OCLC 1435921.
- Cossey, P.J.; Adams, A.E.; Purnell, M.A.; Whiteley, M.J.; Whyte, M.A.; Wright, V.P. (2004). British Lower Carboniferous Stratigraphy. Geological Conservation Review. Peterborough: Joint Nature Conservation Committee. p. 3. ISBN 1-86107-499-9.
- Davydov, Vladimir; Glenister, Brian; Spinosa, Claude; Ritter, Scott; Chernykh, V.; Wardlaw, B.; Snyder, W. (March 1998). "Proposal of Aidaralash as Global Stratotype Section and Point (GSSP) for base of the Permian System". Episodes 21: 11–18. doi:10.18814/epiiugs/1998/v21i1/003. https://stratigraphy.org/gssps/files/asselian.pdf. Retrieved 7 December 2020.
- Dudley, Robert (24 March 1998). "Atmospheric Oxygen, Giant Paleozoic Insects and the Evolution of Aerial Locomotor Performance". The Journal of Experimental Biology 201 (Pt 8): 1043–1050. doi:10.1242/jeb.201.8.1043. PMID 9510518. http://jeb.biologists.org/content/201/8/1043.full.pdf.
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R. A.; Henrissat, B.; Martinez, A. T. et al. (28 June 2012). "The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes". Science 336 (6089): 1715–1719. doi:10.1126/science.1221748. PMID 22745431. Bibcode: 2012Sci...336.1715F.
- Garwood, Russell J.; Edgecombe, Gregory (2011). "Early terrestrial animals, evolution and uncertainty". Evolution: Education and Outreach 4 (3): 489–501. doi:10.1007/s12052-011-0357-y.
- Garwood, Russell J.; Dunlop, Jason A.; Sutton, Mark D. (2009). "High-fidelity X-ray micro-tomography reconstruction of siderite-hosted Carboniferous arachnids". Biology Letters 5 (6): 841–844. doi:10.1098/rsbl.2009.0464. PMID 19656861.
- Garwood, Russell J.; Sutton, Mark D. (2010). "X-ray micro-tomography of Carboniferous stem-Dictyoptera: New insights into early insects". Biology Letters 6 (5): 699–702. doi:10.1098/rsbl.2010.0199. PMID 20392720.
- Haq, B. U.; Schutter, SR (2008). "A Chronology of Paleozoic Sea-Level Changes". Science 322 (5898): 64–68. doi:10.1126/science.1161648. PMID 18832639. Bibcode: 2008Sci...322...64H.
- Heckel, P.H. (2008). "Pennsylvanian cyclothems in Midcontinent North America as far-field effects of waxing and waning of Gondwana ice sheets". Resolving the Late Paleozoic Ice Age in Time and Space. Geological Society of America Special Papers. 441. pp. 275–289. doi:10.1130/2008.2441(19). ISBN 978-0-8137-2441-6.
- Hogan, C. Michael (2010). "Fern". Washington, DC: National council for Science and the Environment. http://www.eoearth.org/article/Fern.
- Kaiser, Sandra (1 April 2009). "The Devonian/Carboniferous boundary stratotype section (La Serre, France) revisited". Newsletters on Stratigraphy 43 (2): 195–205. doi:10.1127/0078-0421/2009/0043-0195. https://www.schweizerbart.de/papers/nos/detail/43/72810/The_Devonian_Carboniferous_boundary_stratotype_section_La_Serre_France_revisited?af=search. Retrieved 7 December 2020.
- Kazlev, M. Alan (1998). "The Carboniferous Period of the Paleozoic Era: 299 to 359 million years ago". http://www.palaeos.com/Paleozoic/Carboniferous/Carboniferous.htm.
- Krulwich, R. (2016). "The Fantastically Strange Origin of Most Coal on Earth". National Geographic. https://www.nationalgeographic.com/science/phenomena/2016/01/07/the-fantastically-strange-origin-of-most-coal-on-earth/.
- Martin, R. Aidan. "A Golden Age of Sharks". http://www.elasmo-research.org/education/evolution/golden_age.htm.
- Menning, M.; Alekseev, A.S.; Chuvashov, B.I.; Davydov, V.I.; Devuyst, F.X.; Forke, H.C.; Grunt, T.A. et al. (2006). "Global time scale and regional stratigraphic reference scales of Central and West Europe, East Europe, Tethys, South China, and North America as used in the Devonian–Carboniferous–Permian Correlation Chart 2003 (DCP 2003)". Palaeogeography, Palaeoclimatology, Palaeoecology 240 (1–2): 318–372. doi:10.1016/j.palaeo.2006.03.058. Bibcode: 2006PPP...240..318M.
- Monastersky, Richard (13 May 1995). "Ancient Animals Got a Rise out of Oxygen". Science News. http://www.highbeam.com/library/docfree.asp?DOCID=1G1:16907261&ctrlInfo=Round20:Mode20b:DocG:Result&ao=. Retrieved 1 May 2018.
- Ogg, Jim (June 2004). "Overview of Global Boundary Stratotype Sections and Points (GSSP's)". http://www.stratigraphy.org/gssp.htm.
- Paproth, Eva; Feist, Raimund; Flajs, Gerd (December 1991). "Decision on the Devonian-Carboniferous boundary stratotype". Episodes 14 (4): 331–336. doi:10.18814/epiiugs/1991/v14i4/004. https://stratigraphy.org/gssps/files/tournaisian.pdf.
- Sahney, S.; Benton, M.J.; Falcon-Lang, H.J. (2010). "Rainforest collapse triggered Pennsylvanian tetrapod diversification in Euramerica". Geology 38 (12): 1079–1082. doi:10.1130/G31182.1. Bibcode: 2010Geo....38.1079S.
- Stanley, S.M. (1999). Earth System History. New York: W.H. Freeman and Company. ISBN 978-0-7167-2882-5.
- Rainer Zangerl and Gerard Ramon Case: Iniopterygia: a new order of Chondrichthyan fishes from the Pennsylvanian of North America. Fieldiana Geology Memoirs, v. 6, Field Museum of Natural History, 1973 Biodiversity Heritage Library (Volltext, engl.)
- Robinson, JM (1990). "Lignin, land plants, and fungi: Biological evolution affecting Phanerozoic oxygen balance.". Geology 18 (7): 607–610. doi:10.1130/0091-7613(1990)018<0607:LLPAFB>2.3.CO;2. Bibcode: 1990Geo....18..607R.
- Scott, A. C.; Glasspool, I. J. (18 July 2006). "The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentration". Proceedings of the National Academy of Sciences 103 (29): 10861–10865. doi:10.1073/pnas.0604090103. PMID 16832054. Bibcode: 2006PNAS..10310861S.
- Verberk, Wilco C.E.P.; Bilton, David T. (July 27, 2011). "Can Oxygen Set Thermal Limits in an Insect and Drive Gigantism?". PLOS ONE 6 (7): e22610. doi:10.1371/journal.pone.0022610. PMID 21818347. Bibcode: 2011PLoSO...622610V.
- Ward, P.; Labandeira, Conrad; Laurin, Michel; Berner, Robert A. (November 7, 2006). "Confirmation of Romer's Gap is a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization". Proceedings of the National Academy of Sciences 103 (45): 16818–16822. doi:10.1073/pnas.0607824103. PMID 17065318. Bibcode: 2006PNAS..10316818W.
- Wells, John (3 April 2008). Longman Pronunciation Dictionary (3rd ed.). Pearson Longman. ISBN 978-1-4058-8118-0.
- "A History of Palaeozoic Forests - Part 2 The Carboniferous coal swamp forests". Westfälische Wilhelms-Universität Münster. https://www.uni-muenster.de/GeoPalaeontologie/Palaeo/Palbot/ewald1.htm.
External links
- "Geologic Time Scale 2004". International Commission on Stratigraphy (ICS). http://www.stratigraphy.org/bak/geowhen/index.html.
- Examples of Carboniferous Fossils
- 60+ images of Carboniferous Foraminifera
- Carboniferous (Chronostratography scale)
 |