Chemistry:Ascomycin
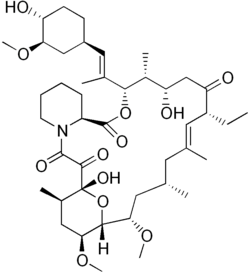 | |
| Clinical data | |
|---|---|
| Other names | 17-ethyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetraone |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C43H69NO12 |
| Molar mass | 792.020 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ascomycin, also called Immunomycin, FR-900520, FK520, is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. It has been researched for the treatment of autoimmune diseases and skin diseases, and to prevent rejection after an organ transplant.[1]
Ascomycin acts by binding to immunophilins, especially macrophilin-12. It appears that Ascomycin inhibits the production of Th1 (interferon- and IL-2) and Th2 (IL-4 and IL-10) cytokines. Additionally, ascomycin preferentially inhibits the activation of mast cells, an important cellular component of the atopic response. Ascomycin produces a more selective immunomodulatory effect in that it inhibits the elicitation phase of allergic contact dermatitis but does not impair the primary immune response when administered systemically.[2]
Ascomycin is produced by the fermentation of certain strains of Streptomyces hygroscopicus.[3]
In fiction
Ascomycin is also the name of a fictional "antiagathic" (anti-aging) drug in James Blish's future history Cities in Flight.[4] and also They shall have stars.
Related compounds
References
- ↑ "Biosynthesis of the immunosuppressants FK506, FK520, and rapamycin involves a previously undescribed family of enzymes acting on chorismate". Proceedings of the National Academy of Sciences of the United States of America 108 (12): 4776–4781. March 2011. doi:10.1073/pnas.1015773108. PMID 21383123.
- ↑ "Ascomycins: promising agents for the treatment of inflammatory skin diseases". Expert Opinion on Investigational Drugs 9 (1): 69–77. January 2000. doi:10.1517/13543784.9.1.69. PMID 11060661.
- ↑ "Enhancement of FK520 production in Streptomyces hygroscopicus by combining traditional mutagenesis with metabolic engineering". Applied Microbiology and Biotechnology 103 (23-24): 9593–9606. December 2019. doi:10.1007/s00253-019-10192-8. PMID 31713669.
- ↑ "Anti-agathic drugs". http://www.technovelgy.com/ct/content.asp?Bnum=1480.
Further reading
- "Ascomycin: an advance in the management of atopic dermatitis". The British Journal of Dermatology 144 (4): 679–681. April 2001. doi:10.1046/j.1365-2133.2001.144004679.x. PMID 11298524.
- "Structure-activity profiles of macrolactam immunosuppressant FK-506 analogues". FEBS Letters 316 (2): 107–113. January 1993. doi:10.1016/0014-5793(93)81196-7. PMID 7678400.
- "The ascomycin macrolactam pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils". The Journal of Allergy and Clinical Immunology 108 (2): 275–280. August 2001. doi:10.1067/mai.2001.116865. PMID 11496246.
- "Comparison of FK-506, rapamycin, ascomycin, and cyclosporine in mouse models of host-versus-graft disease and heterotopic heart transplantation". Annals of the New York Academy of Sciences 685: 55–57. June 1993. doi:10.1111/j.1749-6632.1993.tb35851.x. PMID 7689812.
- "Ascomycins: promising agents for the treatment of inflammatory skin diseases". Expert Opinion on Investigational Drugs 9 (1): 69–77. January 2000. doi:10.1517/13543784.9.1.69. PMID 11060661.
External links
- Exciting New Eczema Treatment Expected This Year By Jane Schwanke, WebMD Medical News March 17, 2000 (San Francisco)
 |

