Chemistry:Dichloro(1,5-cyclooctadiene)palladium
From HandWiki
| |
| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C8H12Cl2Pd | |
| Molar mass | 285.50 g·mol−1 |
| Appearance | yellow solid |
| Density | 2.045 g/cm3 |
| Melting point | 210 °C (410 °F; 483 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
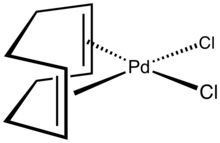
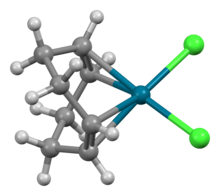
Dichloro(1,5-cyclooctadiene)palladium is the organopalladium compound with the formula PdCl2(C8H12) where C8H12 is cycloocta-1,5-diene (cod) or abbreviated PdCl2(cod). It is a yellow solid that is soluble in chloroform.[1] According to X-ray crystallography, the Pd center is square planar.[2] This complex can be synthesized by reaction of tetrachloropalladate in hydrochloric acid with cycloocta-1,5-diene.[3]
See also
References
- ↑ Shomir Ghosh; Xiang Wang; Anil Guram (2009). "Dichloro(1,5-cyclooctadiene)palladium(II)". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd113.pub2. ISBN 978-0471936237.
- ↑ R. Kumar; A. W. Maverick; F. R. Fronczek; J. R. Doyle; N. C. Baenziger; M. A. M. Howells (1993). "The Pbca polymorph of dichloro(η4-1,5-cyclooctadiene)palladium(II)". Acta Crystallogr. C 49 (10): 1766–1767. doi:10.1107/S0108270193003889.
- ↑ Drew, D.; Doyle, J. R.; Shaver, A. G. (1972). "Cyclic Diolefin Complexes of Platinum and Palladium". Inorganic Syntheses 13: 47–55. doi:10.1002/9780470132449.ch11.
 |

