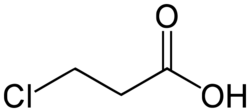
Chemistry:3-Chloropropanoic acid
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C3H5ClO2 |
| Molar mass | 108.52 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 42 °C (108 °F) |
| Boiling point | 204 °C (399 °F) (decomp.) |
| |
| |
| (verify) | |
3-Chloropropanoic acid (also 3-chloropropionic acid) is the organic compound with the formula ClCH2CH2CO2H. A white or colorless solid, it is used as a drug and a synthetic intermediate. The compound is produced by the hydrochlorination of acrylic acid.[1]
With the name UMB66, this compound is a drug used in scientific research. It is structurally related to GHB and binds to the GHB receptor, but has no affinity for GABA receptors.[2] It is also an active ingredient in some herbicide blends.[3] Overdose may cause unconsciousness and/or convulsions.[4]
References
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a22_223.
- ↑ "3-chloropropanoic acid (UMB66): a ligand for the gamma-hydroxybutyric acid receptor lacking a 4-hydroxyl group". Bioorganic & Medicinal Chemistry 12 (7): 1643–1647. April 2004. doi:10.1016/j.bmc.2004.01.025. PMID 15028257.
- ↑ "Degradation Of Herbicide (3-Chloropropionic Acid) By Bacterial Dehalogenases". Proc. KUSTEM 4th Annual Seminar 2005: 586–590. 2005. http://eprints.utm.my/1748/2/FZH.pdf.
- ↑ "Chemical Data Sheet for 3-CHLOROPROPIONIC ACID". NOAA. http://cameochemicals.noaa.gov/chemical/10304.
 |

