Chemistry:Phosphoramide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Phosphoric triamide
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
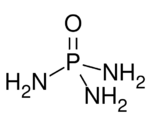
| O=P(NH 2) 3 | |
| Molar mass | 95.042 g·mol−1 |
| Appearance | white solid |
| good | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Phosphoramide is a chemical compound with the molecular formula O=P(NH
2)
3. It is a derivative of phosphoric acid in which each of the hydroxyl groups have been replaced with an amino group. In bulk, the compound is a white solid, soluble in polar solvents.
Chemical properties
Phosphoramide arises from the reaction of phosphoryl chloride with ammonia. In moist air, it hydrolyzes to an ammonium salt:
- 2 H
2O + O=P(NH
2)
3 → [NH
4]+
[PO
2(OH)(NH
2)]−
+ NH
3
It reacts with sodium hydroxide with loss of ammonia:[1]
- NaOH + O=P(NH
2)
3 → Na+
[PO
2(NH
2)
2]−
+ NH
3
The related thiophosphoryl triamide compound S=P(NH
2)
3 was made from the reaction of thiophosphoryl chloride with ammonia.
Phosphoramides
Phosphoramide is also the parent compound for a range of derivatives called phosphoramides.[2] An example compound is the polar solvent hexamethylphosphoramide (HMPA).
References
- ↑ Robert Klement; Otto Koch (1954). "Phosphoroxy‐triamid und Phosphorthio‐triamid". Chemische Berichte 87 (3): 333–340. doi:10.1002/cber.19540870308.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "phosphoramides". doi:10.1351/goldbook.A00484
External links
 |

