Biology:Imine reductase
An imine reductase (IRED) is an enzyme that reduces imines to amines.[1][2] This family of enzymes is employed in the industrial production of amine-containing pharmaceuticals.[3] The IRED enzymes that are found to catalyze both imine formation and imine reduction are called reductive aminases (RedAms).
Function
Imine reduction
Originally discovered in 2010 by screening bacterial strains for reducing activity on 2-methyl-1-pyrroline (2-MPN).[4][5] Based on each member's ability to reduce 2-MPN to (R)- or (S)-2-methylpyrrolidine they are designated as R-selective or S-selective, respectively.[6][7]
Reductive amination
IREDs have been employed to reduce imines formed from ketone-amine mixtures.[1][2] The conversion is not a genuine reductive amination as only the second half of the two-part reaction is catalyzed. In 2017 an IRED was discovered that catalyzed both steps of reductive amination of a wide scope of ketone-amine pairs.[8] These are dubbed reductive aminases (RedAms).[1][2] Engineered RedAms have been employed in industrial processes to support production of pharmaceuticals for clinical trials and commercial manufacturing.[9][10]
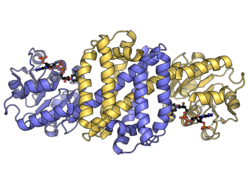
Structure
IREDs are dimeric enzymes with each protomer having an N-terminal Rossmann nucleotide-binding domain and a C-terminal dimerization domain joined by a long interdomain α-helix.[3][11] Each protomer's α-helical dimerization domain wraps around the interdomain helix of its dimer partner forming the substrate-binding cleft above the NAD(P)H cofactor binding site in the Rossmann domain. 3-Hydroxybutyrate dehydrogenases have similar N-terminal nucleotide-binding and C-terminal dimerization domains, but do not share the extensive dimerization interface of IREDs.[12]
See also
References
- ↑ 1.0 1.1 1.2 Mangas-Sanchez, Juan; France, Scott P; Montgomery, Sarah L; Aleku, Godwin A; Man, Henry; Sharma, Mahima; Ramsden, Jeremy I; Grogan, Gideon et al. (2017). "Imine reductases (IREDs)" (in en). Current Opinion in Chemical Biology 37: 19–25. doi:10.1016/j.cbpa.2016.11.022. PMID 28038349. https://linkinghub.elsevier.com/retrieve/pii/S1367593116301880.
- ↑ 2.0 2.1 2.2 Lenz, Maike; Borlinghaus, Niels; Weinmann, Leonie; Nestl, Bettina M. (2017). "Recent advances in imine reductase-catalyzed reactions" (in en). World Journal of Microbiology and Biotechnology 33 (11): 199. doi:10.1007/s11274-017-2365-8. ISSN 0959-3993. PMID 29022156. http://link.springer.com/10.1007/s11274-017-2365-8.
- ↑ 3.0 3.1 Gilio, Amelia K.; Thorpe, Thomas W.; Turner, Nicholas; Grogan, Gideon (2022). "Reductive aminations by imine reductases: from milligrams to tons" (in en). Chemical Science 13 (17): 4697–4713. doi:10.1039/D2SC00124A. ISSN 2041-6520. PMID 35655886. PMC 9067572. http://xlink.rsc.org/?DOI=D2SC00124A.
- ↑ Mitsukura, Koichi; Suzuki, Mai; Tada, Kazuhiro; Yoshida, Toyokazu; Nagasawa, Toru (2010-08-12). "Asymmetric synthesis of chiral cyclic amine from cyclic imine by bacterial whole-cell catalyst of enantioselective imine reductase" (in en). Organic & Biomolecular Chemistry 8 (20): 4533–4535. doi:10.1039/C0OB00353K. ISSN 1477-0520. PMID 20820664. http://xlink.rsc.org/?DOI=C0OB00353K.
- ↑ Mitsukura, Koichi; Suzuki, Mai; Shinoda, Sho; Kuramoto, Tatsuya; Yoshida, Toyokazu; Nagasawa, Toru (2011-09-23). "Purification and Characterization of a Novel ( R )-Imine Reductase from Streptomyces sp. GF3587" (in en). Bioscience, Biotechnology, and Biochemistry 75 (9): 1778–1782. doi:10.1271/bbb.110303. ISSN 0916-8451. PMID 21897027. https://academic.oup.com/bbb/article/75/9/1778-1782/5950175.
- ↑ Scheller, Philipp N.; Fademrecht, Silvia; Hofelzer, Sebastian; Pleiss, Jürgen; Leipold, Friedemann; Turner, Nicholas J.; Nestl, Bettina M.; Hauer, Bernhard (2014-10-13). "Enzyme Toolbox: Novel Enantiocomplementary Imine Reductases" (in en). ChemBioChem 15 (15): 2201–2204. doi:10.1002/cbic.201402213. ISSN 1439-4227. PMID 25163890. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cbic.201402213.
- ↑ Fademrecht, Silvia; Scheller, Philipp N.; Nestl, Bettina M.; Hauer, Bernhard; Pleiss, Jürgen (2016). "Identification of imine reductase-specific sequence motifs" (in en). Proteins: Structure, Function, and Bioinformatics 84 (5): 600–610. doi:10.1002/prot.25008. ISSN 0887-3585. PMID 26857686. https://onlinelibrary.wiley.com/doi/10.1002/prot.25008.
- ↑ Aleku, Godwin A.; France, Scott P.; Man, Henry; Mangas-Sanchez, Juan; Montgomery, Sarah L.; Sharma, Mahima; Leipold, Friedemann; Hussain, Shahed et al. (2017). "A reductive aminase from Aspergillus oryzae" (in en). Nature Chemistry 9 (10): 961–969. doi:10.1038/nchem.2782. ISSN 1755-4330. PMID 28937665. Bibcode: 2017NatCh...9..961A. https://www.nature.com/articles/nchem.2782.
- ↑ Schober, Markus; MacDermaid, Chris; Ollis, Anne A.; Chang, Sandy; Khan, Diluar; Hosford, Joseph; Latham, Jonathan; Ihnken, Leigh Anne F. et al. (2019-09-16). "Chiral synthesis of LSD1 inhibitor GSK2879552 enabled by directed evolution of an imine reductase" (in en). Nature Catalysis 2 (10): 909–915. doi:10.1038/s41929-019-0341-4. ISSN 2520-1158. https://www.nature.com/articles/s41929-019-0341-4.
- ↑ Kumar, Rajesh; Karmilowicz, Michael J.; Burke, Dylan; Burns, Michael P.; Clark, Leslie A.; Connor, Christina G.; Cordi, Eric; Do, Nga M. et al. (2021-09-21). "Biocatalytic reductive amination from discovery to commercial manufacturing applied to abrocitinib JAK1 inhibitor" (in en). Nature Catalysis 4 (9): 775–782. doi:10.1038/s41929-021-00671-5. ISSN 2520-1158. https://www.nature.com/articles/s41929-021-00671-5.
- ↑ Rodríguez-Mata, María; Frank, Annika; Wells, Elizabeth; Leipold, Friedemann; Turner, Nicholas J.; Hart, Sam; Turkenburg, Johan P.; Grogan, Gideon (2013-07-22). "Structure and Activity of NADPH-Dependent Reductase Q1EQE0 from Streptomyces kanamyceticus , which Catalyses the R -Selective Reduction of an Imine Substrate" (in en). ChemBioChem 14 (11): 1372–1379. doi:10.1002/cbic.201300321. ISSN 1439-4227. PMID 23813853. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cbic.201300321.
- ↑ Lenz, Maike; Fademrecht, Silvia; Sharma, Mahima; Pleiss, Jürgen; Grogan, Gideon; Nestl, Bettina M (2018-04-01). "New imine-reducing enzymes from β -hydroxyacid dehydrogenases by single amino acid substitutions" (in en). Protein Engineering, Design and Selection 31 (4): 109–120. doi:10.1093/protein/gzy006. ISSN 1741-0126. PMID 29733377. https://academic.oup.com/peds/article/31/4/109/4992468.
 |