Biology:Plasmin
 Generic protein structure example |
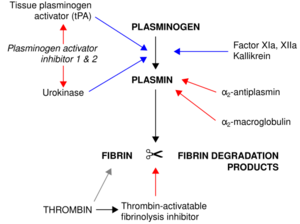
Plasmin is an important enzyme (EC 3.4.21.7) present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the PLG gene.[1]
Function
Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation.[2] It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases.
Plasmin is released as a zymogen called plasminogen (PLG) from the liver into the systemic circulation. Two major glycoforms of plasminogen are present in humans - type I plasminogen contains two glycosylation moieties (N-linked to N289 and O-linked to T346), whereas type II plasminogen contains only a single O-linked sugar (O-linked to T346). Type II plasminogen is preferentially recruited to the cell surface over the type I glycoform. Conversely, type I plasminogen appears more readily recruited to blood clots.
In circulation, plasminogen adopts a closed, activation-resistant conformation. Upon binding to clots, or to the cell surface, plasminogen adopts an open form that can be converted into active plasmin by a variety of enzymes, including tissue plasminogen activator (tPA), urokinase plasminogen activator (uPA), kallikrein, and factor XII (Hageman factor). Fibrin is a cofactor for plasminogen activation by tissue plasminogen activator. Urokinase plasminogen activator receptor (uPAR) is a cofactor for plasminogen activation by urokinase plasminogen activator. The conversion of plasminogen to plasmin involves the cleavage of the peptide bond between Arg-561 and Val-562.[1][3][4][5]
Plasmin cleavage produces angiostatin.
Mechanism of plasminogen activation
Full length plasminogen comprises seven domains. In addition to a C-terminal chymotrypsin-like serine protease domain, plasminogen contains an N-terminal Pan Apple domain (PAp) together with five Kringle domains (KR1-5). The Pan-Apple domain contains important determinants for maintaining plasminogen in the closed form, and the kringle domains are responsible for binding to lysine residues present in receptors and substrates.
The X-ray crystal structure of closed plasminogen reveals that the PAp and SP domains maintain the closed conformation through interactions made throughout the kringle array .[5] Chloride ions further bridge the PAp / KR4 and SP / KR2 interfaces, explaining the physiological role of serum chloride in stabilizing the closed conformer. The structural studies also reveal that differences in glycosylation alter the position of KR3. These data help explain the functional differences between the type I and type II plasminogen glycoforms.[citation needed]
In closed plasminogen, access to the activation bond (R561/V562) targeted for cleavage by tPA and uPA is blocked through the position of the KR3/KR4 linker sequence and the O-linked sugar on T346. The position of KR3 may also hinder access to the activation loop. The Inter-domain interactions also block all kringle ligand-binding sites apart from that of KR-1, suggesting that the latter domain governs pro-enzyme recruitment to targets. Analysis of an intermediate plasminogen structure suggests that plasminogen conformational change to the open form is initiated through KR-5 transiently peeling away from the PAp domain. These movements expose the KR5 lysine-binding site to potential binding partners, and suggest a requirement for spatially distinct lysine residues in eliciting plasminogen recruitment and conformational change respectively.[5]
Mechanism of plasmin inactivation
Plasmin is inactivated by proteins such as α2-macroglobulin and α2-antiplasmin.[6] The mechanism of plasmin inactivation involves the cleavage of an α2-macroglobulin at the bait region (a segment of the aM that is particularly susceptible to proteolytic cleavage) by plasmin. This initiates a conformational change such that the α2-macroglobulin collapses about the plasmin. In the resulting α2-macroglobulin-plasmin complex, the active site of plasmin is sterically shielded, thus substantially decreasing the plasmin's access to protein substrates. Two additional events occur as a consequence of bait region cleavage, namely (i) a h-cysteinyl-g-glutamyl thiol ester of the α2-macroglobulin becomes highly reactive and (ii) a major conformational change exposes a conserved COOH-terminal receptor binding domain. The exposure of this receptor binding domain allows the α2-macroglobulin protease complex to bind to clearance receptors and be removed from circulation.
Pathology
Plasmin deficiency may lead to thrombosis, as the clots are not adequately degraded. Plasminogen deficiency in mice leads to defective liver repair,[7] defective wound healing, reproductive abnormalities.[8] [9]
In humans, a rare disorder called plasminogen deficiency type I (Online Mendelian Inheritance in Man (OMIM) 217090) is caused by mutations of the PLG gene and is often manifested by ligneous conjunctivitis.[10]
A rare missense mutation within the kringle 3 domain of plasminogen, resulting in a novel type of dysplasminogenemia, represents the molecular basis of a subtype of hereditary angioedema with normal C1-inhibitor;[11] the mutation creates a new lysine-binding site within kringle 3 and alters the glycosylation of plasminogen.[11] The mutant plasminogen protein has been shown to be a highly efficient kininogenase that directly releases bradykinin from high- and low-molecular-weight kininogen.[12]
Interactions
Plasmin has been shown to interact with Thrombospondin 1,[13][14] Alpha 2-antiplasmin[15][16] and IGFBP3.[17] Moreover, plasmin induces the generation of bradykinin in mice and humans through high-molecular-weight kininogen cleavage.[18]
References
- ↑ 1.0 1.1 "Entrez Gene: plasminogen". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5340.
- ↑ "Using antifibrinolytics to tackle neuroinflammation". Neural Regeneration Research 15 (12): 2203–2206. December 2020. doi:10.4103/1673-5374.284979. PMID 32594031.
- ↑ "Plasminogen Tochigi: inactive plasmin resulting from replacement of alanine-600 by threonine in the active site". Proceedings of the National Academy of Sciences of the United States of America 79 (20): 6132–6136. October 1982. doi:10.1073/pnas.79.20.6132. PMID 6216475. Bibcode: 1982PNAS...79.6132M.
- ↑ "Molecular cloning and characterization of a full-length cDNA clone for human plasminogen". FEBS Letters 213 (2): 254–260. March 1987. doi:10.1016/0014-5793(87)81501-6. PMID 3030813.
- ↑ 5.0 5.1 5.2 "The X-ray crystal structure of full-length human plasminogen". Cell Reports 1 (3): 185–190. March 2012. doi:10.1016/j.celrep.2012.02.012. PMID 22832192.
- ↑ "Structural studies of plasmin inhibition". Biochemical Society Transactions 47 (2): 541–557. April 2019. doi:10.1042/bst20180211. PMID 30837322.
- ↑ "Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver". Proceedings of the National Academy of Sciences of the United States of America 96 (26): 15143–15148. December 1999. doi:10.1073/pnas.96.26.15143. PMID 10611352. Bibcode: 1999PNAS...9615143B.
- ↑ "Impaired wound healing in mice with a disrupted plasminogen gene". Nature Medicine 2 (3): 287–292. March 1996. doi:10.1038/nm0396-287. PMID 8612226.
- ↑ "Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice". Circulation 92 (9): 2585–2593. November 1995. doi:10.1161/01.cir.92.9.2585. PMID 7586361.
- ↑ "Plasminogen deficiency". Journal of Thrombosis and Haemostasis 5 (12): 2315–2322. December 2007. doi:10.1111/j.1538-7836.2007.02776.x. PMID 17900274.
- ↑ 11.0 11.1 "A missense mutation in the plasminogen gene, within the plasminogen kringle 3 domain, in hereditary angioedema with normal C1 inhibitor". Biochemical and Biophysical Research Communications 498 (1): 193–198. March 2018. doi:10.1016/j.bbrc.2017.12.060. PMID 29548426.
- ↑ "A mechanism for hereditary angioedema caused by a lysine 311-to-glutamic acid substitution in plasminogen". Blood 139 (18): 2816–2829. May 2022. doi:10.1182/blood.2021012945. PMID 35100351.
- ↑ "Complex formation of platelet thrombospondin with plasminogen. Modulation of activation by tissue activator". The Journal of Clinical Investigation 74 (5): 1625–1633. November 1984. doi:10.1172/JCI111578. PMID 6438154.
- ↑ "Thrombospondin interaction with plasminogen. Evidence for binding to a specific region of the kringle structure of plasminogen". Blood 73 (4): 976–982. March 1989. doi:10.1182/blood.V73.4.976.976. PMID 2522013.
- ↑ "On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin". The Journal of Biological Chemistry 254 (18): 9291–9297. September 1979. doi:10.1016/S0021-9258(19)86843-6. PMID 158022.
- ↑ "The reactive site of human alpha 2-antiplasmin". The Journal of Biological Chemistry 262 (13): 6055–6059. May 1987. doi:10.1016/S0021-9258(18)45536-6. PMID 2437112.
- ↑ "Plasminogen binds the heparin-binding domain of insulin-like growth factor-binding protein-3". The American Journal of Physiology 275 (2): E321–E331. August 1998. doi:10.1152/ajpendo.1998.275.2.E321. PMID 9688635.
- ↑ "Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism". Blood 128 (20): 2423–2434. November 2016. doi:10.1182/blood-2016-03-705384. PMID 27531677.
Further reading
- "Hepatic to pancreatic switch defines a role for hemostatic factors in cellular plasticity in mice". Proceedings of the National Academy of Sciences of the United States of America 102 (29): 10182–10187. July 2005. doi:10.1073/pnas.0501691102. PMID 16006527. Bibcode: 2005PNAS..10210182S.
- "Apolipoprotein(a): structure-function relationship at the lysine-binding site and plasminogen activator cleavage site". Biological Chemistry 383 (1): 93–99. January 2002. doi:10.1515/BC.2002.009. PMID 11928826.
- "Plasminogen binding and cancer: promises and pitfalls". Frontiers in Bioscience 8 (6): s294–s304. May 2003. doi:10.2741/1044. PMID 12700073.
External links
- The MEROPS online database for peptidases and their inhibitors: S01.233
- Plasmin at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |