Chemistry:PB-22
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
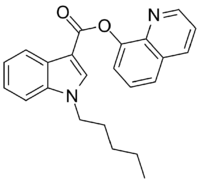
| Formula | C23H22N2O2 |
| Molar mass | 358.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PB-22 (QUPIC, SGT-21 or 1-pentyl-1H-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013.[1][2] PB-22 represents a structurally unique synthetic cannabinoid chemotype, since it contains an ester linker at the indole 3-position, rather than the precedented ketone of JWH-018 and its analogs, or the amide of APICA and its analogs.
PB-22 has an EC50 of 5.1 nM for human CB1 receptors, and 37 nM for human CB2 receptors.[3] PB-22 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, suggesting potent cannabinoid-like activity.[3] The magnitude and duration of hypothermia induced in rats by PB-22 was notably greater than JWH-018, AM-2201, UR-144, XLR-11, APICA, or STS-135, with a reduction of body temperature still observable six hours after dosing.[3] One clinical toxicology study found PB-22 to be the cause of seizures in a human and his dog.[4]
History
PB-22 was originally developed by New Zealand legal highs company Stargate International in 2012 as SGT-21, intended to be a structural hybrid of QMPSB and JWH-018.[5] However, no intellectual property protection was applied for and the compound quickly became subject to widespread grey-market sales outside the control of the inventors.
Detection
A forensic standard of PB-22 is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[6]
Legal status
As of 9 May 2014, PB-22 is no longer legal in New Zealand.[7]
In January 2014, PB-22 was designated as a Schedule I controlled substance in the United States.[8][9]
In Ohio, PB-22 is illegal.[10]
Florida also has banned PB-22.[11]
Since 13 December 2014 it is also illegal in Germany because of adding the substance to the BtMG Anlage II.
As of October 2015 PB-22 is a controlled substance in China.[12]
See also
References
- ↑ "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology 31 (2): 223–240. 2013. doi:10.1007/s11419-013-0182-9.
- ↑ "Pharmacokinetic Approach to Combat the Synthetic Cannabinoid PB-22". ACS Chemical Neuroscience 12 (14): 2573–2579. July 2021. doi:10.1021/acschemneuro.1c00360. PMID 34254505.
- ↑ 3.0 3.1 3.2 "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience 6 (8): 1445–1458. August 2015. doi:10.1021/acschemneuro.5b00107. PMID 25921407. https://zenodo.org/record/47750.
- ↑ "'Crazy Monkey' poisons man and dog: Human and canine seizures due to PB-22, a novel synthetic cannabinoid". Clinical Toxicology 52 (6): 635–638. July 2014. doi:10.3109/15563650.2014.925562. PMID 24905571.
- ↑ "Synthetic cannabinoid receptor agonists: Analytical profiles and development of QMPSB, QMMSB, QMPCB, 2F-QMPSB, QMiPSB, and SGT-233". Drug Testing and Analysis 13 (1): 175–196. January 2021. doi:10.1002/dta.2913. PMID 32880103.
- ↑ Forendex entry, Southern Association of Forensic Scientists
- ↑ "Legal highs pulled from shelves". New Zealand Herald. New Zealand Media and Entertainment. 1 May 2014. http://www.nzherald.co.nz/nz/news/article.cfm?c_id=1&objectid=11247774.
- ↑ "Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22". Journal of Analytical Toxicology 38 (8): 559–562. October 2014. doi:10.1093/jat/bku048. PMID 24876364.
- ↑ "PB-22 and 5F-PB-22". Drug Enforcement Administration, Office of Diversion Control. http://www.deadiversion.usdoj.gov/drug_chem_info/spice/pb22.pdf.
- ↑ Pelzer, Jeremy (April 17, 2014). "Ohio bans two synthetic marijuana drugs sold as "herbal incense"". cleveland.com. http://www.cleveland.com/open/index.ssf/2014/04/ohio_bans_two_synthetic_mariju.html.
- ↑ "Statutes & Constitution :View Statutes : Online Sunshine". Leg.state.fl.us. 1997-05-06. http://www.leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&Search_String=&URL=0800-0899/0893/Sections/0893.03.html.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
 |

