Biology:κ-opioid receptor
 Generic protein structure example |
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.[1][2]
The KOR is a type of opioid receptor that binds the opioid peptide dynorphin as the primary endogenous ligand (substrate naturally occurring in the body).[3] In addition to dynorphin, a variety of natural alkaloids, terpenes and synthetic ligands bind to the receptor. The KOR may provide a natural addiction control mechanism, and therefore, drugs that target this receptor may have therapeutic potential in the treatment of addiction [citation needed].
There is evidence that distribution and/or function of this receptor may differ between sexes.[4][5][6][7]
Distribution
KORs are widely distributed in the brain, spinal cord (substantia gelatinosa), and in peripheral tissues. High levels of the receptor have been detected in the prefrontal cortex, periaqueductal gray, raphe nuclei (dorsal), ventral tegmental area, substantia nigra, dorsal striatum (putamen, caudate), ventral striatum (nucleus accumbens, olfactory tubercle), amygdala, bed nucleus stria terminalis, claustrum, hippocampus, hypothalamus, midline thalamic nuclei, locus coeruleus, spinal trigeminal nucleus, parabrachial nucleus, and solitary nucleus.[8][9]
Subtypes
Based on receptor binding studies, three variants of the KOR designated κ1, κ2, and κ3 have been characterized.[10][11] However, only one cDNA clone has been identified,[12] hence these receptor subtypes likely arise from interaction of one KOR protein with other membrane associated proteins.[13]
Function
Pain
Similarly to μ-opioid receptor (MOR) agonists, KOR agonists are potently analgesic, and have been employed clinically in the treatment of pain. However, KOR agonists also produce side effects such as dysphoria, hallucinations, and dissociation, which has limited their clinical usefulness.[14] Examples of KOR agonists that have been used medically as analgesics include butorphanol, nalbuphine, levorphanol, levallorphan, pentazocine, phenazocine, and eptazocine. Difelikefalin (CR845, FE-202845) and CR665 (FE-200665, JNJ-38488502) are peripherally restricted KOR agonists lacking the CNS side effects of centrally active KOR agonists and are currently under clinical investigation as analgesics.
Consciousness
Centrally active KOR agonists have hallucinogenic or dissociative effects, as exemplified by salvinorin A (the active constituent in Salvia divinorum). These effects are generally undesirable in medicinal drugs. It is thought that the hallucinogenic and dysphoric effects of opioids such as butorphanol, nalbuphine, and pentazocine serve to limit their abuse potential. In the case of salvinorin A, a structurally novel neoclerodane diterpene KOR agonist, these hallucinogenic effects are sought by recreational users, despite the dysphoria experienced by some users. Another KOR agonist with comparable effects is ibogaine, which has possible medical application in addiction treatment. While these KOR agonists possess hallucinogenic and dissociative effects, they are mechanistically and qualitatively different from those of the 5HT2AR agonist psychedelic hallucinogens such as lysergic acid diethylamide (LSD) or psilocybin and those of NMDAR antagonist dissociatives/anesthetics ketamine and phencyclidine.[15]
The claustrum is the region of the brain in which the KOR is most densely expressed.[16][17][18] It has been proposed that this area, based on its structure and connectivity, has "a role in coordinating a set of diverse brain functions", and the claustrum has been elucidated as playing a crucial role in consciousness.[17][18] As examples, lesions of the claustrum in humans are associated with disruption of consciousness and cognition, and electrical stimulation of the area between the insula and the claustrum has been found to produce an immediate loss of consciousness in humans along with recovery of consciousness upon cessation of the stimulation.[18][19] On the basis of the preceding knowledge, it has been proposed that inhibition of the claustrum (as well as, "additionally, the deep layers of the cortex, mainly in prefrontal areas") by activation of KORs in these areas is primarily responsible for the profound consciousness-altering/dissociative hallucinogen effects of salvinorin A and other KOR agonists.[17][18] In addition, it has been stated that "the subjective effects of S. divinorum indicate that salvia disrupts certain facets of consciousness much more than the largely serotonergic hallucinogen [LSD]", and it has been postulated that inhibition of a brain area that is apparently as fundamentally involved in consciousness and higher cognitive function as the claustrum may explain this.[17] However, these conclusions are merely tentative, as "[KORs] are not exclusive to the claustrum; there is also a fairly high density of receptors located in the prefrontal cortex, hippocampus, nucleus accumbens and putamen", and "disruptions to other brain regions could also explain the consciousness-altering effects [of salvinorin A]".[18]
In supplementation of the above, according to Addy et al.:[16]
Theories suggest the claustrum may act to bind and integrate multisensory information, or else to encode sensory stimuli as salient or nonsalient (Mathur, 2014). One theory suggests the claustrum harmonizes and coordinates activity in various parts of the cortex, leading to the seamless integrated nature of subjective conscious experience (Crick and Koch, 2005; Stiefel et al., 2014). Disrupting claustral activity may lead to conscious experiences of disintegrated or unusually bound sensory information, perhaps including synesthesia. Such theories are in part corroborated by the fact that [salvia divinorum], which functions almost exclusively on the KOR system, can cause consciousness to be decoupled from external sensory input, leading to experiencing other environments and locations, perceiving other "beings" besides those actually in the room, and forgetting oneself and one's body in the experience.[16]
Mood, stress, and addiction
The involvement of the KOR in stress, as well as in consequences of chronic stress such as depression, anxiety, anhedonia, and increased drug-seeking behavior, has been made clear.[14] KOR agonists are notably dysphoric and aversive at sufficient doses.[20] The KOR antagonists buprenorphine, as ALKS-5461 (a combination formulation with samidorphan), and CERC-501 (LY-2456302) are currently in clinical development for the treatment of major depressive disorder and substance use disorders.[21] JDTic and PF-4455242 were also under investigation but development was halted in both cases due to toxicity concerns.[21]
The depressive-like behaviors following prolonged morphine abstinence appear to be mediated by upregulation of the KOR/dynorphin system in the nucleus accumbens, as the local application of a KOR antagonist prevented the behaviors.[22] As such, KOR antagonists might be useful for the treatment of depressive symptoms associated with opioid withdrawal.[22]
In a small clinical study, pentazocine, a KOR agonist, was found to rapidly and substantially reduce symptoms of mania in patients with bipolar disorder.[4] It was postulated that the efficacy observed was due to KOR activation-mediated amelioration of excessive dopaminergic signaling in the reward pathways.[4][failed verification]
Others
A variety of other effects of KOR activation are known:
- Activation of the KOR appears to antagonize many of the effects of the MOR, including analgesia, tolerance, euphoria, and memory regulation.[23] Nalorphine and nalmefene are dual MOR antagonists and KOR agonists that have been used clinically as antidotes for opioid overdose, although the specific role and significance of KOR activation in this indication, if any, is uncertain. In any case however, KOR agonists notably do not affect respiratory drive, and hence do not reverse MOR activation-induced respiratory depression.[24]
- KOR agonists suppress itching, and the selective KOR agonist nalfurafine is used clinically as an antipruritic (anti-itch drug).
- Eluxadoline is a peripherally restricted KOR agonist as well as MOR agonist and DOR antagonist that has been approved for the treatment of diarrhea-predominant irritable bowel syndrome. Asimadoline and fedotozine are selective and similarly peripherally restricted KOR agonists that were also investigated for the treatment of irritable bowel syndrome and reportedly demonstrated at least some efficacy for this indication but were ultimately never marketed.
- KOR agonists are known for their characteristic diuretic effects, due to their negative regulation of vasopressin, also known as antidiuretic hormone (ADH).[25]
- KOR agonism is neuroprotective against hypoxia/ischemia.[26]
- The selective KOR agonist U-50488 protected rats against supramaximal electroshock seizures, indicating that KOR agonism may have anticonvulsant effects.[27]
Signal transduction
KOR activation by agonists is coupled to the G protein Gi/G0, which subsequently increases phosphodiesterase activity. Phosphodiesterases break down cAMP, producing an inhibitory effect in neurons.[28][29][30] KORs also couple to inward-rectifier potassium[31] and to N-type calcium ion channels.[32] Recent studies have also demonstrated that agonist-induced stimulation of the KOR, like other G-protein coupled receptors, can result in the activation of mitogen-activated protein kinases (MAPK). These include extracellular signal-regulated kinase, p38 mitogen-activated protein kinases, and c-Jun N-terminal kinases.[33][34][35][36][37][38]
Ligands
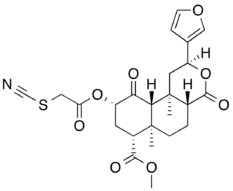
Agonists
The synthetic alkaloid ketazocine[39] and terpenoid natural product salvinorin A[15] are potent and selective KOR agonists. The KOR also mediates the dysphoria and hallucinations seen with opioids such as pentazocine.[40]
- Benzomorphans
- Alazocine– partial agonist
- Bremazocine – highly selective
- 8-Carboxamidocyclazocine
- Cyclazocine – partial agonist
- Ketazocine
- Metazocine – partial agonist
- Pentazocine – partial agonist
- Phenazocine – partial agonist
- 6'-Guanidinonaltrindole (6'-GNTI) – biased ligand: G protein agonist, β-arrestin antagonist
- Butorphan – full agonist
- Butorphanol – partial agonist
- Cyclorphan – full agonist
- Diprenorphine – non-selective, partial agonist
- Etorphine – non-selective
- Levallorphan
- Levomethorphan
- Levorphanol
- Morphine – alkaloid
- Nalbuphine – partial agonist
- Nalfurafine – full agonist, atypical agonist (possibly biased or subtype-selective)
- Nalmefene – partial agonist
- Nalodeine
- Nalorphine – partial agonist
- Norbuprenorphine – partial agonist, peripherally-selective metabolite of buprenorphine
- Norbuprenorphine-3-glucuronide – likely partial agonist, peripherally-selective metabolite of buprenorphine
- Oxilorphan – partial agonist
- Oxycodone – selective for κ2b subtype[41]
- Proxorphan – partial agonist
- Samidorphan – non-selective, weak partial agonist
- Xorphanol – partial agonist
- Asimadoline – peripherally-selective
- BRL-52537
- Eluxadoline
- Enadoline
- GR-89696 – selective for κ2
- ICI-204,448 – peripherally-selective
- ICI-199,441
- LPK-26 – highly selective
- MB-1C-OH [1]
- Niravoline
- N-MPPP [2]
- Spiradoline
- U-50,488
- U-54,494A [3]
- U-69,593
- Peptides (endo-/exogenous)
- CR665 – peripherally-selective [4]
- Difelikefalin (CR845) – peripherally-selective [5]
- Dynorphins (dynorphin A, dynorphin B, big dynorphin)
- Erinacine E
- Menthol
- RB-64 – G protein biased agonist with a bias factor of 96; β-arrestin antagonist[42]
- Salvinorin A – naturally-occurring
- 2-Methoxymethyl salvinorin B[43] – and its ethoxymethyl and fluoroethoxymethyl homologues[44][45]
- Others/unsorted
- Apadoline
- HS665 [6]
- HZ-2
- Ibogaine – alkaloid
- Ketamine (weak)
- Noribogaine – non-selective, biased ligand: G protein agonist, β-arrestin antagonist
- Tifluadom – (atypical) benzodiazepine
- Mirtazapine - partial agonist at high concentrations
- KSC-12-192 - selective, biased ligand: G protein agonist, β-arrestin antagonist
Nalfurafine (Remitch), which was introduced in 2009, is the first selective KOR agonist to enter clinical use.[46][47]
Antagonists
- 5'-Acetamidinoethylnaltrindole (ANTI) – selective [7]
- 5'-Guanidinonaltrindole (5'-GNTI) – selective, long-acting
- 6'-Guanidinonaltrindole (6'-GNTI) – biased ligand: G protein agonist, β-arrestin antagonist
- Amentoflavone – non-selective; naturally-occurring[48]
- AT-076 – non-selective, likely long acting; JDTic analogue
- Aticaprant – selective, short-acting
- Binaltorphimine – selective, long-acting
- BTRX-335140 - selective
- BU09059 – selective, short-acting; JDTic analogue[49]
- Buprenorphine – non-selective; silent antagonist or weak partial agonist, depending on source
- CVL-354 – selective, short-acting[50]
- Dezocine – non-selective; silent antagonist
- DIPPA – selective, long-acting [8]
- JDTic – selective, long-acting
- LY-255582 - non-selective
- LY-2459989 – selective, short-acting
- LY-2795050 – selective, short-acting
- Methylnaltrexone – non-selective
- ML190 – selective [9]
- ML350 – selective, short-acting[49]
- MR-2266 – non-selective
- Naloxone – non-selective
- Naltrexone – non-selective
- Noribogaine – non-selective; naturally-occurring; biased ligand: G protein agonist, β-arrestin antagonist
- Norbinaltorphimine – selective, long-acting
- Pawhuskin A – selective; naturally-occurring[51]
- PF-4455242 – selective, short-acting
- Quadazocine – non-selective; silent antagonist; preference for κ2
- RB-64 (22-thiocyanatosalvinorin A) – G protein biased agonist with a bias factor of 96; β-arrestin antagonist[42]
- Zyklophin – selective peptide antagonist; dynorphin A analogue
- KSC-12-192 - selective, biased ligand: G protein agonist, β-arrestin antagonist
Natural agonists
Mentha spp.
Found in numerous species of mint, (including peppermint, spearmint, and watermint), the naturally-occurring compound menthol is a weak KOR agonist[52] owing to its antinociceptive, or pain blocking, effects in rats. In addition, mints can desensitize a region through the activation of TRPM8 receptors (the 'cold'/menthol receptor).[53]
Salvia divinorum
The key compound in Salvia divinorum, salvinorin A, is known as a powerful, short-acting KOR agonist.[15][54][55]
Ibogaine
Used for the treatment of addiction in limited countries, ibogaine has become an icon of addiction management among certain underground circles. Despite its lack of addictive properties, ibogaine is listed as a Schedule I compound in the US because it is a psychoactive substance, hence it is considered illegal to possess under any circumstances. Ibogaine is also a KOR agonist[56] and this property may contribute to the drug's anti-addictive efficacy.[57]
Mitragyna speciosa
Role in treatment of drug addiction
KOR agonists have been investigated for their therapeutic potential in the treatment of addiction[58] and evidence points towards dynorphin, the endogenous KOR agonist, to be the body's natural addiction control mechanism.[59] Childhood stress/abuse is a well known predictor of drug abuse and is reflected in alterations of the MOR and KOR systems.[60] In experimental "addiction" models the KOR has also been shown to influence stress-induced relapse to drug seeking behavior. For the drug-dependent individual, risk of relapse is a major obstacle to becoming drug-free. Recent reports demonstrated that KORs are required for stress-induced reinstatement of cocaine seeking.[61][62]
One area of the brain most strongly associated with addiction is the nucleus accumbens (NAcc) and striatum while other structures that project to and from the NAcc also play a critical role. Though many other changes occur, addiction is often characterized by the reduction of dopamine D2 receptors in the NAcc.[63] In addition to low NAcc D2 binding,[64][65] cocaine is also known to produce a variety of changes to the primate brain such as increases prodynorphin mRNA in caudate putamen (striatum) and decreases of the same in the hypothalamus while the administration of a KOR agonist produced an opposite effect causing an increase in D2 receptors in the NAcc.[66]
Additionally, while cocaine overdose victims showed a large increase in KORs (doubled) in the NAcc,[67] KOR agonist administration is shown to be effective in decreasing cocaine seeking and self-administration.[68] Furthermore, while cocaine abuse is associated with lowered prolactin response,[69] KOR activation causes a release in prolactin,[70] a hormone known for its important role in learning, neuronal plasticity and myelination.[71]
It has also been reported that the KOR system is critical for stress-induced drug-seeking. In animal models, stress has been demonstrated to potentiate cocaine reward behavior in a kappa opioid-dependent manner.[72][73] These effects are likely caused by stress-induced drug craving that requires activation of the KOR system. Although seemingly paradoxical, it is well known that drug taking results in a change from homeostasis to allostasis. It has been suggested that withdrawal-induced dysphoria or stress-induced dysphoria may act as a driving force by which the individual seeks alleviation via drug taking.[74] The rewarding properties of drug are altered, and it is clear KOR activation following stress modulates the valence of drug to increase its rewarding properties and cause potentiation of reward behavior, or reinstatement to drug seeking. The stress-induced activation of KORs is likely due to multiple signaling mechanisms. The effects of KOR agonism on dopamine systems are well documented, and recent work also implicates the mitogen-activated protein kinase cascade and pCREB in KOR-dependent behaviors.[36][75]
While the predominant drugs of abuse examined have been cocaine (44%), ethanol (35%), and opioids (24%).[76] As these are different classes of drugs of abuse working through different receptors (increasing dopamine directly and indirectly, respectively) albeit in the same systems produce functionally different responses. Conceptually then pharmacological activation of KOR can have marked effects in any of the psychiatric disorders (depression, bipolar disorder, anxiety, etc.) as well as various neurological disorders (i.e. Parkinson's disease and Huntington's disease).[2][77] Not only are genetic differences in dynorphin receptor expression a marker for alcohol dependence but a single dose of a KOR antagonist markedly increased alcohol consumption in lab animals.[78] There are numerous studies that reflect a reduction in self-administration of alcohol,[79] and heroin dependence has also been shown to be effectively treated with KOR agonism by reducing the immediate rewarding effects[80] and by causing the curative effect of up-regulation (increased production) of MORs[81] that have been down-regulated during opioid abuse.
The anti-rewarding properties of KOR agonists are mediated through both long-term and short-term effects. The immediate effect of KOR agonism leads to reduction of dopamine release in the NAcc during self-administration of cocaine[82] and over the long term up-regulates receptors that have been down-regulated during substance abuse such as the MOR and the D2 receptor. These receptors modulate the release of other neurochemicals such as serotonin in the case of MOR agonists and acetylcholine in the case of D2. These changes can account for the physical and psychological remission of the pathology of addiction. The longer effects of KOR agonism (30 minutes or greater) have been linked to KOR-dependent stress-induced potentiation and reinstatement of drug seeking. It is hypothesized that these behaviors are mediated by KOR-dependent modulation of dopamine, serotonin, or norepinephrine and/or via activation of downstream signal transduction pathways.
Of significant note, while KOR activation blocks many of the behavioral and neurochemical responses elicited by drugs of abuse as stated above. These results are indicative of the KOR induced negative affective states counteracting the rewarding effects of drugs of abuse. Implicating the KOR/dynorphin system as an anti-reward system, supported by the role of KOR signaling and stress, mediating both stress-induced potentiation of drug reward and stress-induced reinstatement of seeking behavior.[2][77] This in turn addresses what was thought to be paradoxical above. That is, rather, KOR signaling is activated/upregulated by stress, drugs of abuse and agonist administration - resulting in negative affective state. As such drug addiction is maintained by avoidance of negative affective states manifest in stress, craving, and drug withdrawal.[83] Consistent with KOR induced negative affective states and role in drug addiction, KOR antagonists are efficacious at blocking negative affect induced by drug withdrawal and at decreasing escalated drug intake in pre-clinical trial involving extended drug access.[2][77][76] Clinically there has been little advancement to evaluate the effects of KOR antagonists due to adverse effects and undesirable pharmacological profiles for clinical testing (i.e. long half-life, poor bioavailability). More recently, a selective, high-affinity KOR antagonist LY2456302 was well-tolerated in CUD patients.[84] Showing feasibility a subsequent proof-of-mechanism trial evaluated JNJ-67953964 (previously LY2456302) potential for treating anhedonia in a double-blind, placebo-controlled, randomized trial in patients with anhedonia and a mood or anxiety disorder.[85] The KOR antagonist significantly increased fMRI ventral striatum activation during reward anticipation while accompanied by therapeutic effects on clinical measures of anhedonia, further reinforces the promise of KOR antagonism and proceeding assessment of clinical impact.[85] Additionally a positron emission tomography (PET) study in cocaine use disorder (CUD) patients utilizing a KOR selective agonist [11C]GR103545 radioligand showed CUD individuals with higher KOR availability were more prone to stress-induced relapse.[86] A subsequent PET scan following a three-day cocaine binge showed a decrease in KOR availability, interpreted as increased endogenous dynorphin competing with the radioligand at the KOR binding sites.[86] Taken together these findings are in support of the negative affect state and further implicate the KOR/dynorphin system clinically and therapeutically relevant in humans with CUD. Taken together, in drug addiction the KOR/dynorphin system is implicated as a homeostatic mechanism to counteract the acute effects of drugs of abuse. Chronic drug use and stress up-regulate the system in turn leading to a dysregulated state which induces negative affective states and stress reactivity.[77]
Traditional models of KOR function in drug addiction have postulated that KOR signaling is associated with dysphoria and aversion, thought to underlie the stress-induced exacerbation of addiction. However, recent research in animal models has proposed alternative models, suggesting that KOR-mediated responses may not act directly on negative valence systems but modulate related processes such as novelty processing.[87][88] Studies in humans same to similar conclusions that KORs may modulate various aspects of reward processing in a manner that is independent of the hedonic valence traditionally ascribed to them.[89][90] This broadens the potential understanding of KORs in addiction beyond a unidimensional framework, implicating their role in complex behaviors and treatment approaches that do not align strictly with stress or aversion. These emerging perspectives may inform the development of novel pharmacotherapies targeting KORs for the treatment of substance use disorders, as they highlight the receptor's multifaceted role in addiction.
Interactions
KOR has been shown to interact with sodium-hydrogen antiporter 3 regulator 1,[91][92] ubiquitin C,[93] 5-HT1A receptor,[94] and RGS12.[95]
See also
- δ-opioid receptor
- μ-opioid receptor
- Nociceptin receptor
References
- ↑ "Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol". Alcoholism: Clinical and Experimental Research 41 (8): 1402–1418. August 2017. doi:10.1111/acer.13406. PMID 28425121.
- ↑ 2.0 2.1 2.2 2.3 "Dynorphin/Kappa Opioid Receptor Signaling in Preclinical Models of Alcohol, Drug, and Food Addiction". International Review of Neurobiology 136: 53–88. 2017. doi:10.1016/bs.irn.2017.08.001. ISBN 9780128124734. PMID 29056156.
- ↑ "Selectivity of dynorphin for kappa opioid receptors". Life Sciences 31 (12–13): 1331–4. 1982. doi:10.1016/0024-3205(82)90374-5. PMID 6128656.
- ↑ 4.0 4.1 4.2 "Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction". Frontiers in Neuroscience 9: 466. 2015. doi:10.3389/fnins.2015.00466. PMID 26733781.
- ↑ "Sex differences in kappa opioid pharmacology". Life Sciences 88 (1–2): 2–16. January 2011. doi:10.1016/j.lfs.2010.10.007. PMID 20951148.
- ↑ "Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques". Psychopharmacology 233 (8): 1435–43. April 2016. doi:10.1007/s00213-016-4239-4. PMID 26892380.
- ↑ "PET imaging of kappa opioid receptors and receptor expression quantified in neuron-derived extracellular vesicles in socially housed female and male cynomolgus macaques". Neuropsychopharmacology 48 (2): 410–417. September 2022. doi:10.1038/s41386-022-01444-9. PMID 36100655.
- ↑ "The role of kappa-opioid receptor activation in mediating antinociception and addiction". Acta Pharmacologica Sinica 31 (9): 1065–70. September 2010. doi:10.1038/aps.2010.138. PMID 20729876.
- ↑ "Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications". Trends in Neurosciences 18 (1): 22–9. January 1995. doi:10.1016/0166-2236(95)93946-U. PMID 7535487.
- ↑ "Selective and enantiospecific acylation of kappa opioid receptors by (1S,2S)-trans-2-isothiocyanato-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexy l] benzeneacetamide. Demonstration of kappa receptor heterogeneity". Journal of Medicinal Chemistry 32 (2): 281–3. February 1989. doi:10.1021/jm00122a001. PMID 2536435.
- ↑ "Pharmacological activities of optically pure enantiomers of the kappa opioid agonist, U50,488, and its cis diastereomer: evidence for three kappa receptor subtypes". European Journal of Pharmacology 167 (3): 345–53. August 1989. doi:10.1016/0014-2999(89)90443-3. PMID 2553442. https://deepblue.lib.umich.edu/bitstream/2027.42/27799/1/0000199.pdf.
- ↑ "Isolation of a human kappa opioid receptor cDNA from placenta". Biochemical and Biophysical Research Communications 202 (3): 1431–7. August 1994. doi:10.1006/bbrc.1994.2091. PMID 8060324.
- ↑ "G-protein-coupled receptor heterodimerization modulates receptor function". Nature 399 (6737): 697–700. June 1999. doi:10.1038/21441. PMID 10385123. Bibcode: 1999Natur.399..697J.
- ↑ 14.0 14.1 "The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system". The Journal of Neuroscience 28 (2): 407–14. January 2008. doi:10.1523/JNEUROSCI.4458-07.2008. PMID 18184783.
- ↑ 15.0 15.1 15.2 "Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist". Proceedings of the National Academy of Sciences of the United States of America 99 (18): 11934–9. September 2002. doi:10.1073/pnas.182234399. PMID 12192085. Bibcode: 2002PNAS...9911934R.
- ↑ 16.0 16.1 16.2 "The subjective experience of acute, experimentally-induced Salvia divinorum inebriation". Journal of Psychopharmacology 29 (4): 426–35. April 2015. doi:10.1177/0269881115570081. PMID 25691501.
- ↑ 17.0 17.1 17.2 17.3 "The claustrum's proposed role in consciousness is supported by the effect and target localization of Salvia divinorum". Frontiers in Integrative Neuroscience 8: 20. 2014. doi:10.3389/fnint.2014.00020. PMID 24624064.
- ↑ 18.0 18.1 18.2 18.3 18.4 "The effect of claustrum lesions on human consciousness and recovery of function". Consciousness and Cognition 36: 256–64. November 2015. doi:10.1016/j.concog.2015.06.017. PMID 26186439.
- ↑ "Electrical stimulation of a small brain area reversibly disrupts consciousness". Epilepsy & Behavior 37: 32–5. August 2014. doi:10.1016/j.yebeh.2014.05.027. PMID 24967698.
- ↑ "Association of the kappa-opioid system with alcohol dependence". Molecular Psychiatry 11 (11): 1016–24. November 2006. doi:10.1038/sj.mp.4001882. PMID 16924269.
- ↑ 21.0 21.1 "Antagonists of the kappa opioid receptor". Bioorganic & Medicinal Chemistry Letters 24 (9): 2021–32. May 2014. doi:10.1016/j.bmcl.2014.03.040. PMID 24690494.
- ↑ 22.0 22.1 "Antagonism of κ opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence". Behavioural Brain Research 291: 334–41. September 2015. doi:10.1016/j.bbr.2015.05.053. PMID 26049060.
- ↑ "mu-Opposing actions of the kappa-opioid receptor". Trends in Pharmacological Sciences 19 (3): 94–8. March 1998. doi:10.1016/S0165-6147(98)01169-9. PMID 9584625.
- ↑ Substance Abuse: Inpatient and Outpatient Management for Every Clinician. Springer. 1 December 2014. pp. 181–. ISBN 978-1-4939-1951-2. https://books.google.com/books?id=ms2lBQAAQBAJ&pg=PA181.
- ↑ "Mechanism of diuretic action of U-62,066E, a kappa opioid receptor agonist". European Journal of Pharmacology 160 (2): 229–37. January 1989. doi:10.1016/0014-2999(89)90495-0. PMID 2547626.
- ↑ "Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide". Journal of Cerebral Blood Flow and Metabolism 26 (3): 414–20. March 2006. doi:10.1038/sj.jcbfm.9600196. PMID 16049424.
- ↑ "U50,488, a highly selective kappa opioid: anticonvulsant profile in rats". The Journal of Pharmacology and Experimental Therapeutics 237 (1): 49–53. April 1986. PMID 3007743.
- ↑ "The kappa opioid receptor expressed on the mouse R1.1 thymoma cell line is coupled to adenylyl cyclase through a pertussis toxin-sensitive guanine nucleotide-binding regulatory protein". The Journal of Pharmacology and Experimental Therapeutics 266 (3): 1678–83. September 1993. PMID 8103800.
- ↑ "Relationship between kappa 1 opioid receptor binding and inhibition of adenylyl cyclase in guinea pig brain membranes". Biochemical Pharmacology 45 (1): 207–16. January 1993. doi:10.1016/0006-2952(93)90394-C. PMID 8381004.
- ↑ "Mu-, delta- and kappa-opioid receptor-mediated inhibition of neurotransmitter release and adenylate cyclase activity in rat brain slices: studies with fentanyl isothiocyanate". European Journal of Pharmacology 154 (2): 169–78. September 1988. doi:10.1016/0014-2999(88)90094-5. PMID 2906610. https://zenodo.org/record/1253868.
- ↑ "Kappa-opioid receptors couple to inwardly rectifying potassium channels when coexpressed by Xenopus oocytes". Molecular Pharmacology 47 (3): 551–7. March 1995. PMID 7700253.
- ↑ "The cloned kappa opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells". Neuroscience 63 (4): 1033–40. December 1994. doi:10.1016/0306-4522(94)90570-3. PMID 7700508.
- ↑ "Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade". Journal of Neurochemistry 74 (2): 564–73. February 2000. doi:10.1046/j.1471-4159.2000.740564.x. PMID 10646507.
- ↑ "Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes". The Journal of Biological Chemistry 280 (30): 27662–9. July 2005. doi:10.1074/jbc.M502593200. PMID 15944153.
- ↑ "Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes". The Journal of Biological Chemistry 281 (26): 18081–9. June 2006. doi:10.1074/jbc.M513640200. PMID 16648139.
- ↑ 36.0 36.1 "Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2". NeuroReport 19 (14): 1417–22. September 2008. doi:10.1097/WNR.0b013e32830dd655. PMID 18766023.
- ↑ "Kappa-opioid receptor signals through Src and focal adhesion kinase to stimulate c-Jun N-terminal kinases in transfected COS-7 cells and human monocytic THP-1 cells". The Journal of Pharmacology and Experimental Therapeutics 310 (1): 301–10. July 2004. doi:10.1124/jpet.104.065078. PMID 14996948.
- ↑ "Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase". The Journal of Biological Chemistry 282 (41): 29803–11. October 2007. doi:10.1074/jbc.M705540200. PMID 17702750.
- ↑ "Multiple opiate receptors: [3Hethylketocyclazocine receptor binding and ketocyclazocine analgesia"]. Proceedings of the National Academy of Sciences of the United States of America 77 (6): 3691–4. June 1980. doi:10.1073/pnas.77.6.3691. PMID 6251477. Bibcode: 1980PNAS...77.3691P.
- ↑ "Drug discrimination studies". Drug and Alcohol Dependence 14 (3–4): 263–82. February 1985. doi:10.1016/0376-8716(85)90061-4. PMID 2859972.
- ↑ "Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain". Pain 132 (3): 289–300. December 2007. doi:10.1016/j.pain.2007.03.022. PMID 17467904.
- ↑ 42.0 42.1 "The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo". The Journal of Pharmacology and Experimental Therapeutics 352 (1): 98–109. January 2015. doi:10.1124/jpet.114.216820. PMID 25320048.
- ↑ "2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A". The Journal of Pharmacology and Experimental Therapeutics 324 (3): 1073–83. March 2008. doi:10.1124/jpet.107.132142. PMID 18089845.
- ↑ "Standard protecting groups create potent and selective kappa opioids: salvinorin B alkoxymethyl ethers". Bioorganic & Medicinal Chemistry 16 (3): 1279–86. February 2008. doi:10.1016/j.bmc.2007.10.067. PMID 17981041.
- ↑ "Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats". Psychopharmacology 203 (2): 203–11. April 2009. doi:10.1007/s00213-008-1458-3. PMID 19153716.
- ↑ An Introduction to Medicinal Chemistry. OUP Oxford. 10 January 2013. pp. 657–. ISBN 978-0-19-969739-7. https://books.google.com/books?id=Pj7xJRuhZxUC&pg=PA657.
- ↑ Chemistry of Opioids. Springer. 21 January 2011. pp. 34, 48, 57–60. ISBN 978-3-642-18107-8. https://books.google.com/books?id=eegLBwAAQBAJ&pg=PA34.
- ↑ "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". Journal of Natural Products 70 (8): 1278–82. August 2007. doi:10.1021/np070194x. PMID 17685652.
- ↑ 49.0 49.1 "Characterization of BU09059: a novel potent selective κ-receptor antagonist". ACS Chemical Neuroscience 5 (3): 177–84. March 2014. doi:10.1021/cn4001507. PMID 24410326.
- ↑ "CVL-354". http://adisinsight.springer.com/drugs/800057691.
- ↑ "Stilbenes as κ-selective, non-nitrogenous opioid receptor antagonists". Journal of Natural Products 77 (2): 311–9. February 2014. doi:10.1021/np4009046. PMID 24456556.
- ↑ "Menthol: a natural analgesic compound". Neuroscience Letters 322 (3): 145–8. April 2002. doi:10.1016/S0304-3940(01)02527-7. PMID 11897159.
- ↑ "Mu and kappa opioid receptor agonists antagonize icilin-induced wet-dog shaking in rats". European Journal of Pharmacology 547 (1–3): 101–5. October 2006. doi:10.1016/j.ejphar.2006.07.026. PMID 16945367.
- ↑ "Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa-receptor homology to humans". The Journal of Pharmacology and Experimental Therapeutics 320 (1): 300–6. January 2007. doi:10.1124/jpet.106.112417. PMID 17060493. http://www.sagewisdom.org/butelmanetal2.pdf.
- ↑ "Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations". The Journal of Pharmacology and Experimental Therapeutics 308 (3): 1197–203. March 2004. doi:10.1124/jpet.103.059394. PMID 14718611.
- ↑ "Mechanisms of antiaddictive actions of ibogaine". Annals of the New York Academy of Sciences 844 (1): 214–226. May 1998. doi:10.1111/j.1749-6632.1998.tb08237.x. PMID 9668680. Bibcode: 1998NYASA.844..214G.
- ↑ "Novel Class of Psychedelic Iboga Alkaloids Disrupts Opioid Addiction States" (in en). bioRxiv: 2021.07.22.453441. 2021-07-23. doi:10.1101/2021.07.22.453441.
- ↑ "Possible pharmacotherapy of the opioid kappa receptor agonist for drug dependence". Annals of the New York Academy of Sciences 1025 (1): 404–13. October 2004. doi:10.1196/annals.1316.050. PMID 15542743. Bibcode: 2004NYASA1025..404H.
- ↑ "Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users". Neuropharmacology 55 (1): 41–6. July 2008. doi:10.1016/j.neuropharm.2008.04.019. PMID 18538358.
- ↑ "Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists". The Journal of Pharmacology and Experimental Therapeutics 325 (1): 313–8. April 2008. doi:10.1124/jpet.107.129908. PMID 18203949.
- ↑ "Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats". Psychopharmacology 183 (1): 118–26. November 2005. doi:10.1007/s00213-005-0167-4. PMID 16184376.
- ↑ "Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system". Psychopharmacology 200 (1): 59–70. September 2008. doi:10.1007/s00213-008-1122-y. PMID 18575850.
- ↑ "Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors". Journal of Psychoactive Drugs 32 (Suppl): i-iv, 1–112. November 2000. doi:10.1080/02791072.2000.10736099. PMID 11280926.
- ↑ "Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system". Brain Research 1157: 1–10. July 2007. doi:10.1016/j.brainres.2007.04.074. PMID 17544385.
- ↑ "Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys". Synapse 30 (1): 88–96. September 1998. doi:10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. PMID 9704885.
- ↑ "Role of serotonin in the regulation of the dynorphinergic system by a kappa-opioid agonist and cocaine treatment in rat CNS". Neuroscience 144 (1): 157–64. January 2007. doi:10.1016/j.neuroscience.2006.09.008. PMID 17055175.
- ↑ "D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims". Annals of the New York Academy of Sciences 877 (1): 507–22. June 1999. doi:10.1111/j.1749-6632.1999.tb09286.x. PMID 10415668. Bibcode: 1999NYASA.877..507M.
- ↑ "U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking". Psychopharmacology 144 (4): 339–46. June 1999. doi:10.1007/s002130051016. PMID 10435406.
- ↑ "Relationship of prolactin response to meta-chlorophenylpiperazine with severity of drug use in cocaine dependence". Human Psychopharmacology 21 (6): 367–75. August 2006. doi:10.1002/hup.780. PMID 16915581.
- ↑ "kappa-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D(2)-like receptor agonists". European Journal of Pharmacology 423 (2–3): 243–9. July 2001. doi:10.1016/S0014-2999(01)01121-9. PMID 11448491.
- ↑ "White matter plasticity and enhanced remyelination in the maternal CNS". The Journal of Neuroscience 27 (8): 1812–23. February 2007. doi:10.1523/JNEUROSCI.4441-06.2007. PMID 17314279.
- ↑ "Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses". The Journal of Neuroscience 23 (13): 5674–83. July 2003. doi:10.1523/JNEUROSCI.23-13-05674.2003. PMID 12843270.
- ↑ "Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system". Neuropsychopharmacology 31 (6): 1241–8. June 2006. doi:10.1038/sj.npp.1300872. PMID 16123746.
- ↑ "A role for brain stress systems in addiction". Neuron 59 (1): 11–34. July 2008. doi:10.1016/j.neuron.2008.06.012. PMID 18614026.
- ↑ "Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria". The Journal of Neuroscience 27 (43): 11614–23. October 2007. doi:10.1523/JNEUROSCI.3769-07.2007. PMID 17959804.
- ↑ 76.0 76.1 "The Rise and Fall of Kappa-Opioid Receptors in Drug Abuse Research". Substance Use Disorders. Handbook of Experimental Pharmacology. 258. 2020. pp. 147–165. doi:10.1007/164_2019_268. ISBN 978-3-030-33678-3.
- ↑ 77.0 77.1 77.2 77.3 "Dynorphin/kappa-opioid receptor control of dopamine dynamics: Implications for negative affective states and psychiatric disorders". Brain Research 1713: 91–101. June 2019. doi:10.1016/j.brainres.2018.09.023. PMID 30244022.
- ↑ "A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats". Psychopharmacology 182 (3): 384–92. November 2005. doi:10.1007/s00213-005-0067-7. PMID 16001119.
- ↑ "Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence". Neuropsychopharmacology 33 (3): 643–52. February 2008. doi:10.1038/sj.npp.1301438. PMID 17473837.
- ↑ "Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study". The Journal of Pharmacology and Experimental Therapeutics 284 (1): 151–61. January 1998. PMID 9435173.
- ↑ "Heterologous mu-opioid receptor adaptation by repeated stimulation of kappa-opioid receptor: up-regulation of G-protein activation and antinociception". Journal of Neurochemistry 85 (5): 1171–9. June 2003. doi:10.1046/j.1471-4159.2003.01754.x. PMID 12753076.
- ↑ "U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats". Neuroscience Letters 181 (1–2): 57–60. November 1994. doi:10.1016/0304-3940(94)90559-2. PMID 7898771.
- ↑ "The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse". Psychopharmacology 210 (2): 121–35. June 2010. doi:10.1007/s00213-010-1825-8. PMID 20352414.
- ↑ "Repeated Administration of Opra Kappa (LY2456302), a Novel, Short-Acting, Selective KOP-r Antagonist, in Persons with and without Cocaine Dependence". Neuropsychopharmacology 43 (4): 928. March 2018. doi:10.1038/npp.2017.245. PMID 29422497.
- ↑ 85.0 85.1 "A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia". Nature Medicine 26 (5): 760–768. May 2020. doi:10.1038/s41591-020-0806-7. PMID 32231295.
- ↑ 86.0 86.1 "Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study". Neuropsychopharmacology 44 (10): 1720–1727. September 2019. doi:10.1038/s41386-019-0398-4. PMID 31026862.
- ↑ "Systemic kappa opioid receptor antagonism accelerates reinforcement learning via augmentation of novelty processing in male mice". Neuropsychopharmacology 48 (6): 857–868. May 2023. doi:10.1038/s41386-023-01547-x. PMID 36804487.
- ↑ "Kappa opioid receptors as modulators of novelty processing". Neuropsychopharmacology 48 (6): 848–849. May 2023. doi:10.1038/s41386-023-01561-z. PMID 36922627.
- ↑ "Selective kappa-opioid antagonism ameliorates anhedonic behavior: evidence from the Fast-fail Trial in Mood and Anxiety Spectrum Disorders (FAST-MAS)". Neuropsychopharmacology 45 (10): 1656–1663. September 2020. doi:10.1038/s41386-020-0738-4. PMID 32544925.
- ↑ "A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia". Nature Medicine 26 (5): 760–768. May 2020. doi:10.1038/s41591-020-0806-7. PMID 32231295.
- ↑ "kappa Opioid receptor interacts with Na(+)/H(+)-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na(+)/H(+) exchange independent of G(i)/G(o) proteins". The Journal of Biological Chemistry 279 (24): 25002–9. June 2004. doi:10.1074/jbc.M313366200. PMID 15070904.
- ↑ "Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate". The Journal of Biological Chemistry 277 (30): 27545–52. July 2002. doi:10.1074/jbc.M200058200. PMID 12004055.
- ↑ "Agonist-promoted Lys63-linked polyubiquitination of the human kappa-opioid receptor is involved in receptor down-regulation". Molecular Pharmacology 73 (4): 1319–30. April 2008. doi:10.1124/mol.107.042846. PMID 18212250.
- ↑ "Participation of dorsal periaqueductal gray 5-HT1A receptors in the panicolytic-like effect of the κ-opioid receptor antagonist Nor-BNI". Behavioural Brain Research 327: 75–82. June 2017. doi:10.1016/j.bbr.2017.03.033. PMID 28347824.
- ↑ "Role of RGS12 in the differential regulation of kappa opioid receptor-dependent signaling and behavior". Neuropsychopharmacology 44 (10): 1728–1741. September 2019. doi:10.1038/s41386-019-0423-7. PMID 31141817.
External links
- "Opioid Receptors: κ". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. http://www.iuphar-db.org/GPCR/ReceptorDisplayForward?receptorID=2409.
- kappa+Opioid+Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

