Biology:Virus-like particle
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure.[1][2][3][4] Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera.[5] VLPs have been produced from components of a wide variety of virus families including Parvoviridae (e.g. adeno-associated virus), Retroviridae (e.g. HIV), Flaviviridae (e.g. Hepatitis C virus), Paramyxoviridae (e.g. Nipah) and bacteriophages (e.g. Qβ, AP205).[1] VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.[6][7] VLPs can also refer to structures produced by some LTR retrotransposons (under Ortervirales) in nature. These are defective, immature virions, sometimes containing genetic material, that are generally non-infective due to the lack of a functional viral envelope.[8][9] In addition, wasps produce polydnavirus vectors with pathogenic genes (but not core viral genes) or gene-less VLPs to help control their host.[10][11]
Applications
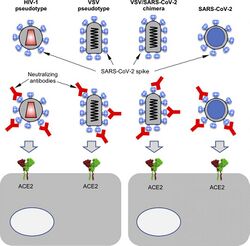
Therapeutic and imaging agents
VLPs are a candidate delivery system for genes or other therapeutics.[12] These drug delivery agents have been shown to effectively target cancer cells in vitro.[13] It is hypothesized that VLPs may accumulate in tumor sites due to the enhanced permeability and retention effect, which could be useful for drug delivery or tumor imaging.[14]
Vaccines
VLPs are useful as vaccines. VLPs contain repetitive, high density displays of viral surface proteins that present conformational viral epitopes that can elicit strong T cell and B cell immune responses.;[15] the particles' small radius of roughly 20-200 nm allows for sufficient draining into lymph nodes. Since VLPs cannot replicate, they provide a safer alternative to attenuated viruses. VLPs were used to develop FDA-approved vaccines for Hepatitis B and human papillomavirus, which are commercially available.
There are currently a selection of vaccines against human papilloma virus (HPV) such as Cervarix by GlaxoSmithKline along with Gardasil and Gardasil-9, produced by Merck & Co. Gardasil consists of recombinant VLPs assembled from the L1 proteins of HPV types 6, 11, 16, and 18 expressed in yeast and is adjuvanted with aluminum hydroxyphosphate sulfate. Gardasil-9 consists of L1 epitopes of 31, 33, 45, 52 and 58 in addition to the listed L1 epitopes found in Gardasil. Cervarix consists of recombinant VLPs assembled from the L1 proteins of HPV types 16 and 18, expressed in insect cells, and is adjuvanted with 3-O-Desacyl-4-monophosphoryl lipid (MPL) A and aluminum hydroxide.[16]
The first VLP vaccine that addresses malaria, Mosquirix, (RTS,S) has been approved by regulators in the EU. It was expressed in yeast. RTS,S is a portion of the Plasmodium falciparum circumsporozoite protein fused to the Hepatitis B surface antigen (RTS), combined with Hepatitis B surface antigen (S), and adjuvanted with AS01 (consisting of (MPL)A and saponin).
Research suggests that VLP vaccines against influenza virus could provide stronger and longer-lasting protection against flu viruses than conventional vaccines.[17] Production can begin as soon as the virus strain is sequenced and can take as little as 12 weeks, compared to 9 months for traditional vaccines. In early clinical trials, VLP vaccines for influenza appeared to provide complete protection against both the Influenza A virus subtype H5N1 and the 1918 flu pandemic.[18] Novavax and Medicago Inc. have run clinical trials of their VLP flu vaccines.[19][20]
VLPs have also been used to develop a pre-clinical vaccine candidate against chikungunya virus.[15]
Lipoparticle technology
The VLP lipoparticle was developed to aid the study of integral membrane proteins.[21] Lipoparticles are stable, highly purified, homogeneous VLPs that are engineered to contain high concentrations of a conformationally intact membrane protein of interest. Integral Membrane proteins are involved in diverse biological functions and are targeted by nearly 50% of existing therapeutic drugs. However, because of their hydrophobic domains, membrane proteins are difficult to manipulate outside of living cells. Lipoparticles can incorporate a wide variety of structurally intact membrane proteins, including G protein-coupled receptors (GPCR)s, ion channels and viral Envelopes. Lipoparticles provide a platform for numerous applications including antibody screening, production of immunogens and ligand binding assays.[22] [23]
Assembly
The understanding of self-assembly of VLPs was once based on viral assembly. This is rational as long as the VLP assembly takes place inside the host cell (in vivo), though the self-assembly event was found in vitro from the very beginning of the study about viral assembly.[24] Study also reveals that in vitro assembly of VLPs competes with aggregation[25] and certain mechanisms exist inside the cell to prevent the formation of aggregates while assembly is ongoing.[26]
Linking targeting groups to VLP surfaces
Attaching proteins, nucleic acids, or small molecules to the VLP surface, such as for targeting a specific cell type or for raising an immune response is useful. In some cases a protein of interest can be genetically fused to the viral coat protein.[27] However, this approach sometimes leads to impaired VLP assembly and has limited utility if the targeting agent is not protein-based. An alternative is to assemble the VLP and then use chemical crosslinkers,[28] reactive unnatural amino acids[29] or SpyTag/SpyCatcher reaction[30][31] in order to covalently attach the molecule of interest. This method is effective at directing the immune response against the attached molecule, thereby inducing high levels of neutralizing antibody and even being able to break tolerance to self-proteins displayed on VLPs.[31]
References
- ↑ 1.0 1.1 "Construction and characterization of virus-like particles: a review". Molecular Biotechnology 53 (1): 92–107. January 2013. doi:10.1007/s12033-012-9598-4. PMID 23001867.
- ↑ "Developments in virus-like particle-based vaccines for infectious diseases and cancer". Expert Review of Vaccines 10 (11): 1569–83. November 2011. doi:10.1586/erv.11.135. PMID 22043956.
- ↑ "NCI Dictionary of Cancer Terms" (in en). 2011-02-02. https://www.cancer.gov/publications/dictionaries/cancer-terms.
- ↑ "Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System". Vaccines 6 (3): 37. July 2018. doi:10.3390/vaccines6030037. PMID 30004398.
- ↑ "Particles associated with Australia antigen in the sera of patients with leukaemia, Down's Syndrome and hepatitis". Nature 218 (5146): 1057–9. June 1968. doi:10.1038/2181057a0. PMID 4231935. Bibcode: 1968Natur.218.1057B.
- ↑ "Virus-like particles production in green plants". Methods 40 (1): 66–76. September 2006. doi:10.1016/j.ymeth.2006.05.020. PMID 16997715.
- ↑ "Escherichia coli-derived virus-like particles in vaccine development". NPJ Vaccines 2 (1): 3. 2017-02-09. doi:10.1038/s41541-017-0006-8. PMID 29263864.
- ↑ "Virus-like particles of the Ty3 retrotransposon assemble in association with P-body components". RNA 12 (1): 94–101. January 2006. doi:10.1261/rna.2264806. PMID 16373495.
- ↑ "Exploring Ty1 retrotransposon RNA structure within virus-like particles". Nucleic Acids Research 41 (1): 463–73. January 2013. doi:10.1093/nar/gks983. PMID 23093595.
- ↑ Burke, Gaelen R.; Strand, Michael R. (2012-01-31). "Polydnaviruses of Parasitic Wasps: Domestication of Viruses To Act as Gene Delivery Vectors" (in en). Insects 3 (1): 91–119. doi:10.3390/insects3010091. PMID 26467950.
- ↑ Leobold, Matthieu; Bézier, Annie; Pichon, Apolline; Herniou, Elisabeth A; Volkoff, Anne-Nathalie; Drezen, Jean-Michel; Abergel, Chantal (July 2018). "The Domestication of a Large DNA Virus by the Wasp Venturia canescens Involves Targeted Genome Reduction through Pseudogenization". Genome Biology and Evolution 10 (7): 1745–1764. doi:10.1093/gbe/evy127. PMID 29931159.
- ↑ "The use of virus-like particles for gene transfer". Current Opinion in Molecular Therapeutics 5 (5): 524–8. October 2003. PMID 14601522.
- ↑ Galaway, F. A. & Stockley, P. G. MS2 viruslike particles: A robust, semisynthetic targeted drug delivery platform. Mol. Pharm. 10, 59–68 (2013).
- ↑ Kovacs, E. W. et al. Dual-surface-modified bacteriophage MS2 as an ideal scaffold for a viral capsid-based drug delivery system. Bioconjug. Chem. 18, 1140–1147 (2007).
- ↑ 15.0 15.1 "A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection". Nature Medicine 16 (3): 334–8. March 2010. doi:10.1038/nm.2105. PMID 20111039.
- ↑ "Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins". Human Vaccines & Immunotherapeutics 11 (5): 1277–92. 2015. doi:10.1080/21645515.2015.1016675. PMID 25751641.
- ↑ "Creating a Mutant Strain of Streptococcus Free of All Integrated Viruses" (Press release). American Society for Microbiology. May 27, 2010. Retrieved June 8, 2010.
- ↑ "Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge". Journal of Virology 83 (11): 5726–34. June 2009. doi:10.1128/JVI.00207-09. PMID 19321609.
- ↑ John Gever (12 September 2010). "ICAAC: High Antibody Titers Seen With Novel Flu Vaccine". http://www.medpagetoday.com/MeetingCoverage/ICAAC/22129.
- ↑ Fouchier, Ron A. M., ed (December 2010). "Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza". PLOS One 5 (12): e15559. doi:10.1371/journal.pone.0015559. PMID 21203523. Bibcode: 2010PLoSO...515559L.
- ↑ "Integral Molecular". http://www.integralmolecular.com/download/INTG-AN-The%20Lipoparticle.pdf.
- ↑ "Virus-like particles as quantitative probes of membrane protein interactions". Biochemistry 47 (27): 6988–90. July 2008. doi:10.1021/bi800540b. PMID 18553929.
- ↑ "Cell-free assay of G-protein-coupled receptors using fluorescence polarization". Journal of Biomolecular Screening 13 (5): 424–9. June 2008. doi:10.1177/1087057108318332. PMID 18567842.
- ↑ "Assembly of a spherical plant virus". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 276 (943): 113–22. November 1976. doi:10.1098/rstb.1976.0102. PMID 13422. Bibcode: 1976RSPTB.276..113A.
- ↑ "Modeling the competition between aggregation and self-assembly during virus-like particle processing". Biotechnology and Bioengineering 107 (3): 550–60. October 2010. doi:10.1002/bit.22821. PMID 20521301.
- ↑ "Chaperone-mediated in vitro assembly of Polyomavirus capsids". Proceedings of the National Academy of Sciences of the United States of America 100 (18): 10477–82. September 2003. doi:10.1073/pnas.1832245100. PMID 12928495. Bibcode: 2003PNAS..10010477C.
- ↑ "Establishment of a yeast-based VLP platform for antigen presentation". Microbial Cell Factories 17 (1): 17. February 2018. doi:10.1186/s12934-018-0868-0. PMID 29402276.
- ↑ "A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses". Vaccine 20 (25–26): 3104–12. August 2002. doi:10.1016/S0264-410X(02)00266-9. PMID 12163261.
- ↑ "Surface functionalization of virus-like particles by direct conjugation using azide-alkyne click chemistry". Bioconjugate Chemistry 22 (3): 376–87. March 2011. doi:10.1021/bc100367u. PMID 21355575.
- ↑ "Plug-and-Display: decoration of Virus-Like Particles via isopeptide bonds for modular immunization". Scientific Reports 6: 19234. January 2016. doi:10.1038/srep19234. PMID 26781591. Bibcode: 2016NatSR...619234B.
- ↑ 31.0 31.1 "Bacterial superglue enables easy development of efficient virus-like particle based vaccines" (in En). Journal of Nanobiotechnology 14 (1): 30. April 2016. doi:10.1186/s12951-016-0181-1. PMID 27117585.
External links
- "Ebola Virus-like Particles Prevent Lethal Ebola Virus Infection". United States Army Medical Research Institute of Infectious Diseases. 2003-12-09. http://www.usamriid.army.mil/press%20releases/bavari_VLP_press_release.pdf.

