Chemistry:Ionophore
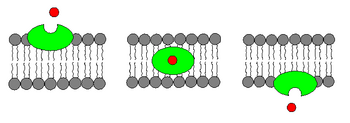
An ionophore is a chemical species that reversibly binds ions.[1] Many ionophores are lipid-soluble entities that transport ions across a cell membrane. "Ionophore" means "ion carrier" as these compounds catalyze ion transport across hydrophobic membranes such as liquid polymeric membranes (carrier-based ion selective electrodes) or lipid bilayers found in the living cells or synthetic vesicles (liposomes).[1]
Some ionophores are synthesized by microorganisms to import ions into their cells. Synthetic ion carriers have also been prepared. Ionophores selective for cations and anions have found many applications in analysis.[2]
The two broad classifications of ionophores synthesized by microorganisms are:
- Carrier ionophores that bind to a particular ion and shield its charge from the surrounding environment. This makes it easier for the ion to pass through the hydrophobic interior of the lipid membrane.[3] An example of a carrier ionophore is valinomycin, a molecule that transports a single potassium cation. Carrier ionophores may be proteins or other molecules.
- Channel formers that introduce a hydrophilic pore into the membrane, allowing ions to pass through without coming into contact with the membrane's hydrophobic interior.[4] An example of a channel former is gramicidin A. Channel forming ionophores are usually large proteins.
Mechanism of action of biologically relevant ionophores
Transmembrane ion concentration gradients (membrane potential) are required for living organisms. Ionophores can disrupt the membrane potential by conducting ions through a lipid membrane in the absence of a protein pore, and thus could exhibit cytotoxic properties. They are produced naturally by a variety of microbes and act as a defense against competing microbes. Many synthetic membrane-spanning ionophores have also been investigated.[5]
Many antibiotics, particularly the macrolide antibiotics, are ionophores. Some exhibit high affinities for Na+, others high affinities for K+.[6] The structure of the sodium and potassium complexes of antibiotics have been verified by X-ray crystallography.[7]
Ionophores have been used to modify the permeability of biological membranes toward certain ions. Additionally, some ionophores are used as antibiotics and/or as growth-enhancing feed additives for certain animals, such as cattle (see monensin) and chickens.[8]
Synthetic ionophores
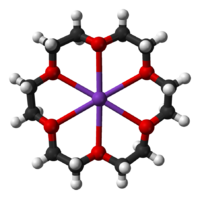
Many synthetic ionophores are based on crown ethers, cryptands, and calixarenes. These synthetic species are often macrocyclic.[6] Some synthetic agents are not macrocyclic, e.g., carbonyl cyanide-p-trifluoromethoxyphenylhydrazone. Even simple organic compounds, such as phenols, exhibit ionophoric properties. The majority of synthetic receptors used in the carrier-based anion-selective electrodes employ transition elements or metalloids as anion carriers, although simple organic urea- and thiourea based receptors are known.
Zinc ionophores
Zinc ionophores transport extracelluar Zn2+ ions across a cell membrane, and have been studied for their anti-viral and anti-cancer activities.[9][10]
Quinoline derivatives:
- Chloroquine (4-Aminoquinoline)[11]
- Clioquinol (8-Hydroxyquinoline)[10]
- Diiodohydroxyquinoline (Quinoline)[12]
- PBT2 (8-Hydroxyquinoline analog)[13]
Terpenoids and flavonols:
Other compounds:
- Epigallocatechin gallate[15]
- Pyrithione (ZnHPT)[14][10][16]
- Pyrrolidine dithiocarbamate (PDTC)[17]
- Zincophorin[10]
Zinc ionophores have been shown to inhibit replication of various viruses in vitro:
- Coxsackievirus[14][17]
- Equine viral arteritis[18]
- Hepatitis C virus[19]
- Herpes simplex virus[20]
- Human coronavirus 229E[21]
- Human Immunodeficiency Virus[22][23]
- Mengovirus[14][17]
- MERS coronavirus[21]
- Rhinovirus[14]
- SARS coronavirus[18][21]
- Zika virus[24][25]
List of representative biological ionophores
With the ion(s) they act upon:
- Beauvericin (Ca2+, Ba2+)
- Calcimycine or A23187 (Mn2+, Ca2+, Mg2+)
- Cezomycin
- Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (H+)
- Enniatin (ammonium)
- Gramicidin A (H+, Na+, K+)
- 2,4-Dinitrophenol (DNP) (H+)
- Ionomycin (Ca2+)
- Lasalocid (K+, Na+, Ca2+, Mg2+)[26]
- Monensin (Na+, H+)
- Nigericin (K+, H+, Pb2+)
- Nonactin (ammonium ionophore I)
- Salinomycin (K+)
- Tetronasin
- Valinomycin (potassium ionophore I)
- Narasin
Crystal structures have been determined for many of the alkali metal complexes formed by these compounds.[27]
See also
- Siderophore - Fe3+ binding compounds, found in microbes and grasses
References
- ↑ 1.0 1.1 Bakker E1, Bühlmann P, Pretsch E. (1997). "Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics". Chem. Rev. 97 (8): 3083–3132. doi:10.1021/cr940394a. PMID 11851486.
- ↑ Bühlmann P1, Pretsch E, Bakker E. (1998). "Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors". Chem. Rev. 98 (4): 1593–1688. doi:10.1021/cr970113+. PMID 11848943.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Ionophore". doi:10.1351/goldbook.IT06772
- ↑ "Ionophores - MeSH Result". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=mesh&Cmd=ShowDetailView&TermToSearch=68007476&ordinalpos=1&itool=EntrezSystem2.PEntrez.Mesh.Mesh_ResultsPanel.Mesh_RVDocSum.
- ↑ Rodríguez-Vázquez, Nuria; Fuertes, Alberto; Amorín, Manuel; Granja, Juan R. (2016). "Chapter 14. Bioinspired Artificial Sodium and Potassium Ion Channels". in Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel. The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. 16. Springer. pp. 485–556. doi:10.1007/978-3-319-21756-7_14.
- ↑ 6.0 6.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Steinrauf, L. K.; Hamilton, J. A.; Sabesan, M. N. (1982). "Crystal structure of valinomycin-sodium picrate. Anion effects on valinomycin-cation complexes". Journal of the American Chemical Society 104 (15): 4085–4091. doi:10.1021/ja00379a008.
- ↑ Kabel, Marcus; Christine Simmons (2007-11-20). "USDA Revokes OK for Tyson Chicken Labels". Archived from the original on November 24, 2007. https://web.archive.org/web/20071124025150/http://ap.google.com/article/ALeqM5iYfYJmjwYMb-6RiqjCbJ481k5xjgD8T16P5O0. Retrieved 2007-11-20.
- ↑ Ishida, Tsuneo (21 March 2019). "Review on The Role of Zn2+ Ions in Viral Pathogenesis and the Effect of Zn2+ Ions for Host Cell-Virus Growth Inhibition". American Journal of Biomedical Science & Research 2 (1): 28–37. doi:10.34297/AJBSR.2019.02.000566.
- ↑ 10.0 10.1 10.2 10.3 Ding, Wei-Qun; Lind, Stuart E. (November 2009). "Metal ionophores - An emerging class of anticancer drugs". IUBMB Life 61 (11): 1013–1018. doi:10.1002/iub.253. PMID 19859983.
- ↑ Xue, Jing; Moyer, Amanda; Peng, Bing; Wu, Jinchang; Hannafon, Bethany N.; Ding, Wei-Qun; Ho, Yuan-Soon (1 October 2014). "Chloroquine Is a Zinc Ionophore". PLOS ONE 9 (10): e109180. doi:10.1371/journal.pone.0109180. PMID 25271834.
- ↑ Aggett, P.J.; Delves, H.T.; Harries, J.T.; Bangham, A.D. (March 1979). "The possible role of Diodoquin as a zinc ionophore in the treatment of acrodermatitis enteropathica". Biochemical and Biophysical Research Communications 87 (2): 513–517. doi:10.1016/0006-291X(79)91825-4. PMID 375935.
- ↑ Bohlmann, Lisa; De Oliveira, David M. P.; El-Deeb, Ibrahim M.; Brazel, Erin B.; Harbison-Price, Nichaela; Ong, Cheryl-lynn Y.; Rivera-Hernandez, Tania; Ferguson, Scott A. et al. (11 December 2018). "Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance". mBio 9 (6). doi:10.1128/mBio.02391-18. PMID 30538186.
- ↑ 14.0 14.1 14.2 14.3 14.4 Krenn, B. M.; Gaudernak, E.; Holzer, B.; Lanke, K.; Van Kuppeveld, F. J. M.; Seipelt, J. (1 January 2009). "Antiviral Activity of the Zinc Ionophores Pyrithione and Hinokitiol against Picornavirus Infections". Journal of Virology 83 (1): 58–64. doi:10.1128/JVI.01543-08. PMID 18922875.
- ↑ 15.0 15.1 Dabbagh-Bazarbachi, Husam; Clergeaud, Gael; Quesada, Isabel M.; Ortiz, Mayreli; O’Sullivan, Ciara K.; Fernández-Larrea, Juan B. (31 July 2014). "Zinc Ionophore Activity of Quercetin and Epigallocatechin-gallate: From Hepa 1-6 Cells to a Liposome Model". Journal of Agricultural and Food Chemistry 62 (32): 8085–8093. doi:10.1021/jf5014633. PMID 25050823.
- ↑ Magda, D.; Lecane, P.; Wang, Z.; Hu, W.; Thiemann, P.; Ma, X.; Dranchak, P. K.; Wang, X. et al. (1 July 2008). "Synthesis and Anticancer Properties of Water-Soluble Zinc Ionophores". Cancer Research 68 (13): 5318–5325. doi:10.1158/0008-5472.CAN-08-0601. PMID 18593933.
- ↑ 17.0 17.1 17.2 Lanke, K.; Krenn, B. M.; Melchers, W. J. G.; Seipelt, J.; van Kuppeveld, F. J. M. (1 April 2007). "PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells". Journal of General Virology 88 (4): 1206–1217. doi:10.1099/vir.0.82634-0. PMID 17374764.
- ↑ 18.0 18.1 te Velthuis, Aartjan J. W.; van den Worm, Sjoerd H. E.; Sims, Amy C.; Baric, Ralph S.; Snijder, Eric J.; van Hemert, Martijn J.; Andino, Raul (4 November 2010). "Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture". PLOS Pathogens 6 (11): e1001176. doi:10.1371/journal.ppat.1001176. PMID 21079686.
- ↑ Mizui, Tomokazu; Yamashina, Shunhei; Tanida, Isei; Takei, Yoshiyuki; Ueno, Takashi; Sakamoto, Naoya; Ikejima, Kenichi; Kitamura, Tsuneo et al. (17 September 2009). "Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy". Journal of Gastroenterology 45 (2): 195–203. doi:10.1007/s00535-009-0132-9. PMID 19760134.
- ↑ Qiu, Min; Chen, Yu; Chu, Ying; Song, Siwei; Yang, Na; Gao, Jie; Wu, Zhiwei (October 2013). "Zinc ionophores pyrithione inhibits herpes simplex virus replication through interfering with proteasome function and NF-κB activation". Antiviral Research 100 (1): 44–53. doi:10.1016/j.antiviral.2013.07.001. PMID 23867132.
- ↑ 21.0 21.1 21.2 de Wilde, Adriaan H.; Jochmans, Dirk; Posthuma, Clara C.; Zevenhoven-Dobbe, Jessika C.; van Nieuwkoop, Stefan; Bestebroer, Theo M.; van den Hoogen, Bernadette G.; Neyts, Johan et al. (August 2014). "Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture". Antimicrobial Agents and Chemotherapy 58 (8): 4875–4884. doi:10.1128/AAC.03011-14. PMID 24841269.
- ↑ TSAI, WEN-PO; NARA, PETER L.; KUNG, HSIANG-FU; OROSZLAN, STEPHEN (April 1990). "Inhibition of Human Immunodeficiency Virus Infectivity by Chloroquine". AIDS Research and Human Retroviruses 6 (4): 481–489. doi:10.1089/aid.1990.6.481. PMID 1692728.
- ↑ Romanelli, Frank; Smith, Kelly; Hoven, Ardis (1 August 2004). "Chloroquine and Hydroxychloroquine as Inhibitors of Human Immunodeficiency Virus (HIV-1) Activity". Current Pharmaceutical Design 10 (21): 2643–2648. doi:10.2174/1381612043383791. PMID 15320751.
- ↑ Delvecchio, Rodrigo; Higa, Luiza; Pezzuto, Paula; Valadão, Ana; Garcez, Patrícia; Monteiro, Fábio; Loiola, Erick; Dias, André et al. (29 November 2016). "Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models". Viruses 8 (12): 322. doi:10.3390/v8120322. PMID 27916837.
- ↑ Li, Chunfeng; Zhu, Xingliang; Ji, Xue; Quanquin, Natalie; Deng, Yong-Qiang; Tian, Min; Aliyari, Roghiyh; Zuo, Xiangyang et al. (October 2017). "Chloroquine, a FDA-approved Drug, Prevents Zika Virus Infection and its Associated Congenital Microcephaly in Mice". EBioMedicine 24: 189–194. doi:10.1016/j.ebiom.2017.09.034. PMID 29033372.
- ↑ Antonenko, YN; Yaguzhinsky, LS (18 February 1988). "The ion selectivity of nonelectrogenic ionophores measured on a bilayer lipid membrane: nigericin, monensin, A23187 and lasalocid A.". Biochimica et Biophysica Acta (BBA) - Biomembranes 938 (2): 125–30. doi:10.1016/0005-2736(88)90151-4. PMID 19927398.
- ↑ Katsuyuki, Aoki; Kazutaka, Murayama; Hu, Ning-Hai (2016). "Chapter 3, section 5. Naturally Occurring Antibiotic IOnophore Complexes". in Astrid, Sigel; Helmut, Sigel; Roland K.O., Sigel. The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. 16. Springer. pp. 74–92. doi:10.1007/978-3-319-21756-7_3.
External links
- Fluka ionophores for ion-selective electrodes
- Medical Information database Reference.MD
- Structures and Properties of Naturally Occurring Polyether Antibiotics, J. Rutkowski, B. Brzezinski; open access review article
- Polyether ionophores—promising bioactive molecules for cancer therapy, A. Huczyński; open access review article[yes|permanent dead link|dead link}}]
 |