Chemistry:Gold(II) sulfate
From HandWiki
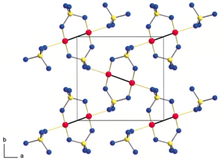 Structure of gold(II) sulfate(Red spheres: Au; Yellow spheres: S; Blue spheres: O)
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| Properties | |
| Au 2(SO 4) 2 | |
| Molar mass | 293.03 g/mol |
| Appearance | Red crystals |
| Density | 5.51 g/cm3 |
| Structure | |
| Orthorhombic | |
| Pbca | |
a = 854.9 pm, b = 824.9 pm, c = 1001.4 pm
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gold(II) sulfate is the chemical compound with the formula AuSO
4 or more correctly Au
2(SO
4)
2. This compound was previously thought to be a mixed-valent compound as AuIAuIII(SO4)2. But later, it was shown that it contained the diatomic cation, Au4+2 which made it the first simple inorganic gold(II) compound. The bond distance between the gold atoms in the diatomic cation is 249 pm.[1][2]
Production and properties
Gold(II) sulfate is produced by reaction of sulfuric acid and gold(III) hydroxide. Gold(II) sulfate is unstable in air and oxidizes to hydrogen disulfoaurate(III)(HAu(SO
4)
2).[1]
References
- ↑ 1.0 1.1 Wickleder, Mathias S. (2001). "AuSO4: A True Gold(II) Sulfate with an Au24+ Ion". Journal of Inorganic and General Chemistry 627 (9): 2112–2114. doi:10.1002/1521-3749(200109)627:9<2112::AID-ZAAC2112>3.0.CO;2-2.
- ↑ Wickleder, Mathias S. (2007). Devillanova, Francesco A.. ed. Handbook of chalcogen chemistry: new perspectives in sulfur, selenium and tellurium. Royal Society of Chemistry. pp. 359–361. ISBN 978-0-85404-366-8. https://books.google.com/books?id=IvGnUAaSqOsC&pg=PA359.
 |

