Physics:Open microfluidics
Microfluidics refers to the flow of fluid in channels or networks with at least one dimension on the micron scale.[1][2] In open microfluidics, also referred to as open surface microfluidics or open-space microfluidics, at least one boundary confining the fluid flow of a system is removed, exposing the fluid to air or another interface such as a second fluid.[1][3][4]
Types of open microfluidics
Open microfluidics can be categorized into various subsets. Some examples of these subsets include open-channel microfluidics, paper-based, and thread-based microfluidics.[1][5][6]
Open-channel microfluidics
In open-channel microfluidics, a surface tension-driven capillary flow occurs and is referred to as spontaneous capillary flow (SCF).[1][7] SCF occurs when the pressure at the advancing meniscus is negative.[1] The geometry of the channel and contact angle of fluids has been shown to produce SCF if the following equation is true.
[math]\displaystyle{ {pf \over pw}\lt cos(\theta) }[/math]
Where pf is the free perimeter of the channel (i.e., the interface not in contact with the channel wall), and pw is the wetted perimeter[8] (i.e., the walls in contact with the fluid), and θ is the contact angle of the fluid on the material of the device.[1][5]
Paper-based microfluidics
Paper-based microfluidics utilizes the wicking ability of paper for functional readouts.[9][10] Paper-based microfluidics is an attractive method because paper is cheap, easily accessible, and has a low environmental impact. Paper is also versatile because it is available in various thicknesses and pore sizes.[9] Coatings such as wax have been used to guide flow in paper microfluidics.[11] In some cases, dissolvable barriers have been used to create boundaries on the paper and control the fluid flow.[12] The application of paper as a diagnostic tool has shown to be powerful because it has successfully been used to detect glucose levels,[13] bacteria,[14] viruses,[15] and other components in whole blood.[16] Cell culture methods within paper have also been developed.[17][18] Lateral flow immunoassays, such as those used in pregnancy tests, are one example of the application of paper for point of care or home-based diagnostics.[19] Disadvantages include difficulty of fluid retention and high limits of detection.
Thread-based microfluidics
Thread-based microfluidics, an offshoot from paper-based microfluidics, utilizes the same capillary based wicking capabilities.[20] Common thread materials include nitrocellulose, rayon, nylon, hemp, wool, polyester, and silk.[21] Threads are versatile because they can be woven to form specific patterns.[22] Additionally, two or more threads can converge together in a knot bringing two separate ‘streams’ of fluid together as a reagent mixing method.[23] Threads are also relatively strong and difficult to break from handling which makes them stable over time and easy to transport.[21] Thread-based microfluidics has been applied to 3D tissue engineering and analyte analysis.[24][20]
Capillary filaments in open microfluidics
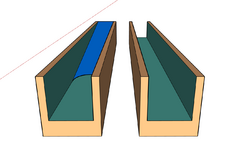
Open capillary microfluidics are channels that expose fluids to open air by excluding the ceiling and/or floor of the channel.[5] Rather than rely on using pumps or syringes to maintain flow, open capillary microfluidics uses surface tension to facilitate the flow.[25] The elimination of and infusion source reduces the size of the device and associated apparatus, along with other aspects that could obstruct their use. The dynamics of capillary-driven flow in open microfluidics are highly reliant on two types of geometric channels commonly known as either rectangular U-grooves or triangular V-grooves.[26][25] The geometry of the channels dictates the flow along the interior walls fabricated with various ever-evolving processes.[7]
Capillary filaments in U-groove
Rectangular open-surface U-grooves are the easiest type of open microfluidic channel to fabricate. This design can maintain the same order of magnitude velocity in comparison to V-groove.[27][26][28] Channels are made of glass or high clarity glass substitutes such as polymethyl methacrylate (PMMA),[25] polycarbonate (PC), or cyclic olefin copolymer (COC).[25][citation needed] To eliminate the remaining resistance after etching, channels are given hydrophilic treatment using oxygen plasma or deep reactive-ion etching(DRIE).[29][30][31]
Capillary filaments in V-groove
V-groove, unlike U-groove, allows for a variety of velocities depending on the groove angle.[28] V-grooves with sharp groove angle result in the interface curvature at the corners explained by reduced Concus-Finn conditions.[32] In a perfect inner corner of a V-groove, the filament will advance indefinitely in the groove allowing the formation of capillary filament depending on the wetting conditions.[33] The width of the groove plays an important role in controlling the fluid flow. The narrower the V-groove is, the better the capillary flow of liquids is even for highly viscous liquids such as blood; this effect has been used to produce an autonomous assay.[5][34] The fabrication of a V-groove is more difficult than a U-groove as it poses a higher risk for faulty construction, since the corner has to be tightly sealed.[29]
Advantages
One of the main advantages of open microfluidics is ease of accessibility which enables intervention (i.e., for adding or removing reagents) to the flowing liquid in the system.[35] Open microfluidics also allows simplicity of fabrication thus eliminating the need to bond surfaces. When one of the boundaries of a system is removed, a larger liquid-gas interface results, which enables liquid-gas reactions.[1][36] Open microfluidic devices enable better optical transparency because at least one side of the system is not covered by the material which can reduce autofluorescence during imaging.[37] Further, open systems minimize and sometimes eliminate bubble formation, a common problem in closed systems.[1]
In closed system microfluidics, the flow in the channels is driven by pressure via pumps (syringe pumps), valves (trigger valves), or electrical field.[38] An example of one of these methods for achieving low flow rates using temperature-controlled evaporation has been described for an open microfluidics system, allowing for long incubation hours for biological applications and requiring small sample volumes.[39] Open system microfluidics enable surface-tension driven flow in channels thereby eliminating the need for external pumping methods.[35][40] For example, some open microfluidic devices consist of a reservoir port and pumping port that can be filled with fluid using a pipette.[1][5][35] Eliminating external pumping requirements lowers cost and enables device use in all laboratories with pipettes.[36]
Materials Solutions
Thankfully, while many problems exist with PDMS, many solutions have also been developed. To address the negative hydrophobicity and porosity that PDMS exhibits, researchers have started to use coatings such as BSA[41] (bovine serum albumin) or charged molecules[42] to create a layer between the native PDMS and the cells. Other researchers have successfully employed several of the Pluronic surfactants,[43] a tri-block copolymer that has two hydrophilic blocks surrounding a hydrophobic core often used to increase the hydrophilic nature of numerous substrates, and even borosilicate glass coatings[44] to address the hydrophobicity problem. Interestingly, treatment with either of the prior two compounds can result in prevention of non-specific protein adsorption, as they (and other coatings) form stable adsorption interactions with the PDMS, which aides in reducing PDSM interference with cell culture media. These compounds and materials can affect surface properties and should be carefully tested to note the impact on cultured cells. Researchers developed 3D scaffolding systems to mimic in vivo environments so that more cells and cell types can grow[45] in an effort to address the problem that not all cell types can grow on PDMS. Like coating the PDMS, 3D scaffolding systems employ alternatives materials like ECM (extracellular matrix) proteins[46] so rather than not binding the native PDMS, cells are more likely to bind to the proteins. Lastly, researchers have addressed the permeability of PDMS to water vapor using some elegant solutions. For example, a portion of the microfluidic system can be designated for humidification and cast in PDMS, or other material like glass.
Disadvantages
Some drawbacks of open microfluidics include evaporation,[47] contamination,[48] and limited flow rate.[4] Open systems are susceptible to evaporation which can greatly affect readouts when fluid volumes are on the microscale.[47] Additionally, due to the nature of open systems, they are more susceptible to contamination than closed systems.[48] Cell culture and other methods where contamination or small particulates are a concern must be carefully performed to prevent contamination. Lastly, open systems have a limited flow rate because induced pressures cannot be used to drive flow.[4]
Materials
Polydimethylsiloxane (PDMS) is an ideal material to fabricate microfluidic devices for cell culture applications due to several advantageous properties such as low processing costs, ease of manufacture, rapid prototyping, ease of surface modification, and cellular non-toxicity.[49][42] While there are several benefits that arise from using native Polydimethylsiloxane (PDMS), there are also some drawbacks that researchers must account for in their experiments. First, PDMS is both hydrophobic and porous, meaning that small molecules or other hydrophobic molecules can be adsorbed onto it.[50] Such molecules include anything from methyl- or alkyl-containing molecules,[51] and even certain dyes like Nile Red.[52] Researchers identified in 2008 that plasma could be used to reduce the hydrophobicity of PDMS, though it returned about two weeks after treatment.[53] Some researchers postulate that integrating removable polycaprolactone (PCL) fiber-based electrospun scaffolds under NaOH treatment enhances hydrophilicity as well as mitigating hydrophobicity, while promoting more efficient cell communication.[54] Another problem that arises with PDMS is that it can interfere with the media that circulates in the channels. Incomplete curing of PDMS channels can lead to PDMS leaching into the media[55] and, even when complete curing takes place, components of the media can still unintentionally attach to free hydrophobic sites on the PDMS walls.[56] Yet another problem arises with the gas permeability of PDMS. Most researchers take advantage of this to oxygenate both the PDMS and the circulating media, but this trait also makes the microfluidic system especially vulnerable to water vapor loss. Lastly, not all cell types can grow, or will grow at the same levels, on native PDMS.[57] For instance, high levels of rapid cell death in two fibroblast types grown on native PDMS were observed as early as 1994, which posed problems for the widespread use of PDMS in microfluidic cell culture.
Applications
Like many microfluidic technologies, open system microfluidics has been applied to nanotechnology, biotechnology, fuel cells, and point of care (POC) testing.[1][4][58] For cell-based studies, open-channel microfluidic devices enable access to cells for single cell probing within the channel.[59] Other applications include capillary gel electrophoresis, water-in-oil emulsions, and biosensors for POC systems.[3][60][61] Suspended microfluidic devices, open microfluidic devices where the floor of the device is removed, have been used to study cellular diffusion and migration of cancer cells.[5] Suspended and rail-based microfluidics have been used for micropatterning and studying cell communication.[1]
Materials Solutions Applications
Applications of these solutions are still in use today, as seen by the following examples. In 2014, Lei et al was testing the impedance of human oral cancer cells in the presence of cisplatin, a known anti-cancer drug, by molding the cells into a 3D scaffolding.[62] The authors had noted from previous studies that cellular impedance could be correlated to cellular viability and proliferation in 2D cell culture and hoped to translate that correlation into 3D cell culture. Using agarose to create the 3D scaffolding, the researchers measured the growth and proliferation of human oral cancer cells in the presence and absence of cisplatin using fluorescent DNA assays and observed that there was indeed a correlation like that observed in 2D model. Not only did this prove that principles from 2D cell culture could be translated to 3D open microfluidic cell culture, but it also potentially lays the foundation for a more personalized treatment plan for cancer patients. They postulated that future developments could transform this method into an assay that could test patient cancer cell response to known anti-cancer drugs.
Another group used a similar method, but instead of creating a 3D scaffolding, they employed several different PDMS coatings to determine the best option for studying cancer stem cells.[63] The group looked at BSA and ECM proteins and found that, while their experimental evidence supported BSA as the best coating for circulating cancer cells (CSC’s), phenotypic changes did occur to the cells (namely, elongation), but did not impact the cells’ ability to perform normal cell functions. A key caveat to note here is that BSA is not a blanket solution that works for every cell type- different coatings work better or worse for certain cell types[46] and these differences should be considered when developing an experiment.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Open microfluidics. Hoboken, NJ: Wiley. 2016. ISBN 9781118720936. OCLC 953661963.
- ↑ "The origins and the future of microfluidics". Nature 442 (7101): 368–73. July 2006. doi:10.1038/nature05058. PMID 16871203. Bibcode: 2006Natur.442..368W.
- ↑ 3.0 3.1 "Trends in microfluidics with complex fluids". ChemPhysChem 4 (12): 1291–8. December 2003. doi:10.1002/cphc.200300847. PMID 14714376.
- ↑ 4.0 4.1 4.2 4.3 "Microfluidics in the "open space" for performing localized chemistry on biological interfaces". Angewandte Chemie 51 (45): 11224–40. November 2012. doi:10.1002/anie.201201798. PMID 23111955.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Suspended microfluidics". Proceedings of the National Academy of Sciences of the United States of America 110 (25): 10111–6. June 2013. doi:10.1073/pnas.1302566110. PMID 23729815. Bibcode: 2013PNAS..11010111C.
- ↑ "Toward practical application of paper-based microfluidics for medical diagnostics: state-of-the-art and challenges". Lab on a Chip 17 (7): 1206–1249. March 2017. doi:10.1039/c6lc01577h. PMID 28251200.
- ↑ 7.0 7.1 "Dynamics of Capillary-Driven Flow in Open Microchannels". The Journal of Physical Chemistry C 115 (38): 18761–18769. 2011-09-07. doi:10.1021/jp2065826. ISSN 1932-7447.
- ↑ "Wetted perimeter" (in en), Wikipedia, 2018-11-27, https://en.wikipedia.org/w/index.php?title=Wetted_perimeter&oldid=870799531, retrieved 2019-04-16
- ↑ 9.0 9.1 "Paper and Fiber-Based Bio-Diagnostic Platforms: Current Challenges and Future Needs". Applied Sciences 7 (8): 863. 2017-08-22. doi:10.3390/app7080863.
- ↑ "Rapid light transmittance measurements in paper-based microfluidic devices". Sensing and Bio-Sensing Research 5: 55–61. 2015-09-01. doi:10.1016/j.sbsr.2015.07.005. ISSN 2214-1804.
- ↑ "Automatic Paper Chromatography". Analytical Chemistry 21 (9): 1123–1125. September 1949. doi:10.1021/ac60033a032. ISSN 0003-2700.
- ↑ "Controlled reagent transport in disposable 2D paper networks". Lab on a Chip 10 (7): 918–20. April 2010. doi:10.1039/b919614e. PMID 20300678.
- ↑ "Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis". Analytical Chemistry 80 (10): 3699–707. May 2008. doi:10.1021/ac800112r. PMID 18407617.
- ↑ "Paper-based ELISA to rapidly detect Escherichia coli". Talanta 145: 2–5. December 2015. doi:10.1016/j.talanta.2015.07.051. PMID 26459436.
- ↑ "Cellulose-based diagnostic devices for diagnosing serotype-2 dengue fever in human serum". Advanced Healthcare Materials 3 (2): 187–96. February 2014. doi:10.1002/adhm.201470008. PMID 23843297.
- ↑ "Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices". Lab on a Chip 12 (2): 274–80. January 2012. doi:10.1039/c1lc20803a. PMID 22094609.
- ↑ "Paper-based cell culture microfluidic system". BioChip Journal 9 (2): 97–104. 2015-03-18. doi:10.1007/s13206-015-9202-7. ISSN 1976-0280.
- ↑ "Cell chemotaxis on paper for diagnostics". Analytical Chemistry 87 (11): 5505–10. June 2015. doi:10.1021/acs.analchem.5b00726. PMID 25938457.
- ↑ "A Chemically Patterned Microfluidic Paper-based Analytical Device (C-µPAD) for Point-of-Care Diagnostics". Scientific Reports 7 (1): 1188. April 2017. doi:10.1038/s41598-017-01343-w. PMID 28446756. Bibcode: 2017NatSR...7.1188L.
- ↑ 20.0 20.1 "Surface Modified Thread-Based Microfluidic Analytical Device for Selective Potassium Analysis". Analytical Chemistry 88 (10): 5331–7. May 2016. doi:10.1021/acs.analchem.6b00633. PMID 27077212. https://zenodo.org/record/3766254.
- ↑ 21.0 21.1 "Thread as a matrix for biomedical assays". ACS Applied Materials & Interfaces 2 (6): 1722–8. June 2010. doi:10.1021/am1002266. PMID 20496913.
- ↑ "Thread as a versatile material for low-cost microfluidic diagnostics". ACS Applied Materials & Interfaces 2 (1): 1–6. January 2010. doi:10.1021/am9006148. PMID 20356211.
- ↑ "Flow control concepts for thread-based microfluidic devices". Biomicrofluidics 5 (1): 14105. March 2011. doi:10.1063/1.3567094. PMID 21483659.
- ↑ "A toolkit of thread-based microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics". Microsystems & Nanoengineering 2 (1): 16039. 2016-07-18. doi:10.1038/micronano.2016.39. PMID 31057832.
- ↑ 25.0 25.1 25.2 25.3 "Spontaneous capillary flow in curved, open microchannels" (in en). Microfluidics and Nanofluidics 20 (7): 100. July 2016. doi:10.1007/s10404-016-1766-6. ISSN 1613-4982.
- ↑ 26.0 26.1 "Metastable capillary filaments in rectangular cross-section open microchannels". AIMS Biophysics 1 (1): 31–48. 2014. doi:10.3934/biophy.2014.1.31. ISSN 2377-9098.
- ↑ "Suspended microflows between vertical parallel walls". Microfluidics and Nanofluidics 18 (5–6): 919–929. 2014-09-18. doi:10.1007/s10404-014-1482-z. ISSN 1613-4982.
- ↑ 28.0 28.1 "Filling kinetics of liquids in nanochannels as narrow as 27 nm by capillary force". Journal of Colloid and Interface Science 293 (1): 151–7. January 2006. doi:10.1016/j.jcis.2005.06.037. PMID 16023663. Bibcode: 2006JCIS..293..151H. http://doc.rero.ch/record/16966/files/Han_Anpan._-_Filling_kinetics_of_liquids_in_nanochannels_20100112.pdf.
- ↑ 29.0 29.1 "Groovy drops: effect of groove curvature on spontaneous capillary flow". Langmuir 23 (16): 8406–10. July 2007. doi:10.1021/la700473m. PMID 17608505.
- ↑ Process Challenges for Integration of Copper Interconnects with Low-k Dielectrics. ECS Transactions. 35. Montreal, QC, Canada. 2011. pp. 687–699. doi:10.1149/1.3572313. Bibcode: 2011ECSTr..35d.687G.
- ↑ Advanced Etch Tool for High Etch Rate Deep Reactive Ion Etching in Silicon Micromachining Production Environment. Berlin Heidelberg: Springer. 2001. pp. 229–236. ISBN 978-3-642-62124-6.
- ↑ "A general condition for spontaneous capillary flow in uniform cross-section microchannels". Microfluidics and Nanofluidics 16 (4): 779–785. 2013-11-06. doi:10.1007/s10404-013-1270-1. ISSN 1613-4982.
- ↑ "Solder wetting kinetics in narrow V-grooves". Acta Materialia 45 (12): 5337–5345. December 1997. doi:10.1016/s1359-6454(97)00205-x. ISSN 1359-6454. Bibcode: 1997AcMat..45.5337Y.
- ↑ "Coagulation dynamics of a blood sample by multiple scattering analysis". Journal of Biomedical Optics 16 (5): 057001–057001–9. May 2011. doi:10.1117/1.3573813. PMID 21639579. Bibcode: 2011JBO....16e7001F.
- ↑ 35.0 35.1 35.2 "Droplet Behavior in Open Biphasic Microfluidics". Langmuir 34 (18): 5358–5366. May 2018. doi:10.1021/acs.langmuir.8b00380. PMID 29692173.
- ↑ 36.0 36.1 "Surface-directed liquid flow inside microchannels". Science 291 (5506): 1023–6. February 2001. doi:10.1126/science.291.5506.1023. PMID 11161212. Bibcode: 2001Sci...291.1023Z.
- ↑ "Assessment of enhanced autofluorescence and impact on cell microscopy for microfabricated thermoplastic devices". Analytical Chemistry 85 (1): 44–9. January 2013. doi:10.1021/ac3034773. PMID 23249264.
- ↑ "The present and future role of microfluidics in biomedical research". Nature 507 (7491): 181–9. March 2014. doi:10.1038/nature13118. PMID 24622198. Bibcode: 2014Natur.507..181S.
- ↑ "Continuous flow in open microfluidics using controlled evaporation". Lab on a Chip 5 (12): 1355–9. December 2005. doi:10.1039/B510044E. PMID 16286965.
- ↑ The Motion of a Surface by Its Mean Curvature. (MN-20). Princeton: Princeton University Press. 2015-01-31. doi:10.1515/9781400867431. ISBN 9781400867431.
- ↑ "Studying cancer stem cell dynamics on PDMS surfaces for microfluidics device design". Scientific Reports 3 (1): 2332. December 2013. doi:10.1038/srep02332. PMID 23900274. Bibcode: 2013NatSR...3E2332Z.
- ↑ 42.0 42.1 "Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices". Biosensors & Bioelectronics 63: 218–231. January 2015. doi:10.1016/j.bios.2014.07.029. PMID 25105943.
- ↑ "Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices". Microfluidics and Nanofluidics 7 (3): 291–306. September 2009. doi:10.1007/s10404-009-0443-4. PMID 20357909.
- ↑ "Internal modification of poly(dimethylsiloxane) microchannels with a borosilicate glass coating". Langmuir 24 (16): 9154–61. August 2008. doi:10.1021/la801317x. PMID 18652427.
- ↑ "Recent advances in microfluidic 3D cellular scaffolds for drug assays" (in en). TrAC Trends in Analytical Chemistry 87: 19–31. February 2017. doi:10.1016/j.trac.2016.11.009.
- ↑ 46.0 46.1 "Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane)". Langmuir 20 (26): 11684–91. December 2004. doi:10.1021/la048562+. PMID 15595798.
- ↑ 47.0 47.1 "Evaporation from open microchannel grooves". Lab on a Chip 14 (4): 771–8. February 2014. doi:10.1039/c3lc50892g. PMID 24345870.
- ↑ 48.0 48.1 "Development of hydrogel microtubes for microbe culture in open environment". 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2015. August 2015. pp. 5896–5899. doi:10.1109/EMBC.2015.7319733. ISBN 978-1-4244-9271-8.
- ↑ "Gas permeation through rubbery polymer nano-corrugated membranes". Scientific Reports 8 (1): 6345. April 2018. doi:10.1038/s41598-018-24551-4. PMID 29679013. Bibcode: 2018NatSR...8.6345F.
- ↑ "Biocompatibility and reduced drug absorption of sol-gel-treated poly(dimethyl siloxane) for microfluidic cell culture applications". Analytical Chemistry 82 (21): 8954–60. November 2010. doi:10.1021/ac101870s. PMID 20936785.
- ↑ "PDMS and its suitability for analytical microfluidic devices". 2006 International Conference of the IEEE Engineering in Medicine and Biology Society. 2006. IEEE. August 2006. pp. 2486–9. doi:10.1109/IEMBS.2006.260465. ISBN 978-1-4244-0032-4.
- ↑ "PDMS absorption of small molecules and consequences in microfluidic applications". Lab on a Chip 6 (12): 1484–6. December 2006. doi:10.1039/B612140C. PMID 17203151.
- ↑ "Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—An SEM investigation" (in en). Sensors and Actuators B: Chemical 123 (1): 368–373. 2007-04-10. doi:10.1016/j.snb.2006.08.037.
- ↑ "Integrating Microstructured Electrospun Scaffolds in an Open Microfluidic System for in Vitro Studies of Human Patient-Derived Primary Cells". ACS Biomaterials Science & Engineering 6 (6): 3649–3663. June 2020. doi:10.1021/acsbiomaterials.0c00352. PMID 33463182.
- ↑ "Biological implications of polydimethylsiloxane-based microfluidic cell culture". Lab on a Chip 9 (15): 2132–9. August 2009. doi:10.1039/b903043c. PMID 19606288.
- ↑ "Surface modification for PDMS-based microfluidic devices". Electrophoresis 33 (1): 89–104. January 2012. doi:10.1002/elps.201100482. PMID 22128067.
- ↑ "In vitro study of the intrinsic toxicity of synthetic surfaces to cells". Journal of Biomedical Materials Research 28 (6): 667–75. June 1994. doi:10.1002/jbm.820280603. PMID 8071377.
- ↑ "Droplet-based Biosensing for Lab-on-a-Chip, Open Microfluidics Platforms". Biosensors 6 (2): 14. April 2016. doi:10.3390/bios6020014. PMID 27089377.
- ↑ ""Microcanals" for micropipette access to single cells in microfluidic environments". Lab on a Chip 4 (5): 420–4. October 2004. doi:10.1039/b404956j. PMID 15472724.
- ↑ "Open-channel, water-in-oil emulsification in paper-based microfluidic devices". Lab on a Chip 17 (8): 1436–1441. April 2017. doi:10.1039/c7lc00114b. PMID 28322402.
- ↑ "Open microfluidic gel electrophoresis: Rapid and low cost separation and analysis of DNA at the nanoliter scale". Electrophoresis 38 (13–14): 1764–1770. July 2017. doi:10.1002/elps.201700001. PMID 28426159.
- ↑ "Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip". Biosensors & Bioelectronics 51: 16–21. January 2014. doi:10.1016/j.bios.2013.07.031. PMID 23920091.
- ↑ "Studying cancer stem cell dynamics on PDMS surfaces for microfluidics device design". Scientific Reports 3 (1): 2332. 2013-07-31. doi:10.1038/srep02332. PMID 23900274. Bibcode: 2013NatSR...3E2332Z.
 |