Chemistry:Goitrin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
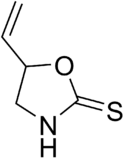
5-Vinyl-1,3-oxazolidine-2-thione | |
| Other names
Goitrin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H7NOS | |
| Molar mass | 129.18 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Goitrin is an organosulfur compound classified as a derivative of oxazolidine and as a cyclic thiocarbamate. It reduces the production of thyroid hormones such as thyroxine.[1] It is found in cruciferous vegetables such as cabbage, brussels sprouts and rapeseed oil,[2] and is formed by the hydrolysis of a glucosinolate: progoitrin or 2-hydroxy-3-butenyl glucosinolate. The unstable isothiocyanate (2-hydroxy-3-butenyl isothiocyanate) derived from the latter glucosinolate spontaneously cyclizes to goitrin, because the hydroxy group is situated in proximity to the isothiocyanate group (allowing a five-membered ring to be formed). Hence, the oxygen in the molecule stems from the hydroxy group of the original unstable isothiocyanate. Plants containing this specific glucosinolate (or glucosinolates such as glucobrassicin and sinalbin which liberate thiocyanate ion) have goitrogenic potential due to the goitrin and thiocyanate they contain. However, they do not seem to alter thyroid function in humans at realistic amounts in the diet.[3]
See also
- Goitrogen
References
- ↑ "Preliminary Observations on the Effect of Dietary Brussels Sprouts on Thyroid Function". Hum Toxicol 5 (1): 15–19. January 1986. doi:10.1177/096032718600500104. PMID 2419242.
- ↑ "Goitrin — a nitrosatable constituent of plant foodstuffs". Experientia 40 (5): 452–453. May 1984. doi:10.1007/BF01952381. PMID 6723906.
- ↑ "A review of mechanisms underlying anticarcinogenicity by brassica vegetables". Chem. Biol. Interact. 103 (2): 79–129. February 1997. doi:10.1016/S0009-2797(96)03745-3. PMID 9055870.
 |

