Chemistry:Hexamethylcyclotrisiloxane
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| [(CH 3) 2SiO] 3 | |
| Molar mass | 222.462 g·mol−1 |
| Appearance | Colorless or white solid |
| Density | 1.02 g/cm3 |
| Melting point | 64 °C (147 °F; 337 K) |
| Boiling point | 134 °C (273 °F; 407 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H315, H319, H335 | |
| P210, P240, P241, P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
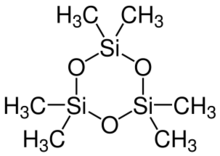
Hexamethylcyclotrisiloxane, also known as D3 and D3, is the organosilicon compound with the formula [(CH
3)
2SiO]
3. It is a colorless or white volatile solid. It finds limited use in organic chemistry. The larger tetrameric and pentameric siloxanes, respectively octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane, are of significant industrial interest,[1] whereas 1,000–10,000 tonnes per year of the trimer is manufactured and/or imported in the European Economic Area.[2]
Structure and reactions
Hexamethylcyclotrisiloxane adopts a planar structure and is considered strained.[3][4] It reacts with organolithium reagents to give, after hydrolysis, dimethylsilanols:
- [(CH
3)
2SiO]
3 + 3 RLi → 3 RSi(CH
3)
2OLi - RSi(CH
3)
2OLi + H
2O → RSi(CH
3)
2OH + LiOH
Safety and environmental considerations
The LD50 for the related pentamer (D5) is >50 g/kg in rats.[1]
See also
References
- ↑ 1.0 1.1 Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057. ISBN 3-527-30673-0.
- ↑ "InfoCard – Hexamethylcyclotrisiloxane". ECHA. https://echa.europa.eu/substance-information/-/substanceinfo/100.007.970.
- ↑ Scott E. Denmark; Christopher R. Butler (2007). eEROS. doi:10.1002/047084289X.rn00784. ISBN 978-0-471-93623-7.
- ↑ Brook, Michael A. (2000). Silicon in Organic, Organometallic and Polymer Chemistry. New York: Wiley. p. 262. ISBN 0-471-19658-4.
 |

