Chemistry:Meclonazepam
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
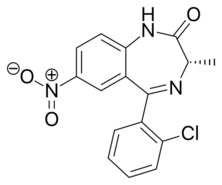
| Formula | C16H12ClN3O3 |
| Molar mass | 329.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Meclonazepam[1] ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam.[2] It has sedative and anxiolytic actions like those of other benzodiazepines,[3] and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.[4]
Meclonazepam was never used as medicine and instead appeared online as a designer drug.[5][6][7][8]
Legal Issues
United Kingdom
In the UK, meclonazepam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[9]
See also
References
- ↑ Szente A, "Benzodiazepine derivatives", US patent 4031078, issued 21 June 1977, assigned to Hoffmann La Roche Inc.
- ↑ The Lundbeck Institute. "Meclonazepam". Psychotropics. Lundbeck. http://www.psychotropics.dk/moleculeView/default.aspx?ID=1426&Catalogtype=A&ChapterID=1&Thissortorder=23.
- ↑ "Initial study of methylclonazepam in generalized anxiety disorder. Evidence for greater power in the cross-over design". Psychopharmacology 87 (2): 130–135. 1985. doi:10.1007/bf00431795. PMID 3931136. https://orbi.uliege.be/bitstream/2268/259489/1/Ansseau1985_Article_InitialStudyOfMethylclonazepam.pdf.
- ↑ "Central effects in man of the novel schistosomicidal benzodiazepine meclonazepam". European Journal of Clinical Pharmacology 29 (1): 105–108. 1985. doi:10.1007/bf00547377. PMID 4054198.
- ↑ "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry 408 (13): 3571–3591. May 2016. doi:10.1007/s00216-016-9439-6. PMID 27071765.
- ↑ "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis 9 (4): 640–645. April 2017. doi:10.1002/dta.2003. PMID 27366870.
- ↑ "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis 10 (8): 1258–1269. March 2018. doi:10.1002/dta.2387. PMID 29582576. https://rke.abertay.ac.uk/en/publications/527a634d-decc-4d3a-bdca-08659bb13ed6.
- ↑ "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology 40 (2): 349–356. July 2022. doi:10.1007/s11419-022-00616-y. PMID 36454409.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2017". http://www.legislation.gov.uk/uksi/2017/634/contents/made.
Further reading
- "Experimentally promising antischistosomal drugs: a review of some drug candidates not reaching the clinical use". Parasitology Research 105 (4): 899–906. October 2009. doi:10.1007/s00436-009-1546-2. PMID 19588166.
 |

