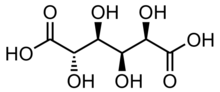
Chemistry:Saccharic acid
| |
| Names | |
|---|---|
| IUPAC name
D-glucaric acid
| |
| Other names
(2R,3S,4S,5S)-2,3,4,5-tetrahydroxyhexanedioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10O8 | |
| Molar mass | 210.1388 |
| Melting point | 125-126 °C (decomposes) |
| 912 g/L[1] | |
| Acidity (pKa) | pKa1 = 3.01[2] pKa2 = 3.94[2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| HH228Script error: No such module "Preview warning".Category:GHS errors, HH314Script error: No such module "Preview warning".Category:GHS errors | |
| PP210Script error: No such module "Preview warning".Category:GHS errors, PP240Script error: No such module "Preview warning".Category:GHS errors, PP241Script error: No such module "Preview warning".Category:GHS errors, PP260Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP264+P265Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP301+P330+P331Script error: No such module "Preview warning".Category:GHS errors, PP302+P361+P354Script error: No such module "Preview warning".Category:GHS errors, PP304+P340Script error: No such module "Preview warning".Category:GHS errors, PP305+P354+P338Script error: No such module "Preview warning".Category:GHS errors, PP316Script error: No such module "Preview warning".Category:GHS errors, PP317Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP363Script error: No such module "Preview warning".Category:GHS errors, PP370+P378Script error: No such module "Preview warning".Category:GHS errors, PP405Script error: No such module "Preview warning".Category:GHS errors, PP501Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Saccharic acid or glucaric acid[3] is a chemical compound with the formula C6H10O8. It is an aldaric acid, naturally occurring in fruits and vegetables.[4]
The salts of saccharic acid are called saccharates or glucarates.
Synthesis
Saccharic acid can be prepared by oxidizing both the aldehydic and primary alcohol groups in an aldose, such as glucose, forming the dicarboxylic acid.[5][6] A suitable reagent for this transformation is boiling 30% nitric acid, resulting in a yield of 50% to 65%.[6] This reaction was first described by German chemist Heinrich Kiliani in 1925.[7]
Uses
Detergents
The sodium salt has found use in dishwasher detergents, where it acts as a chelating agent for calcium and magnesium ions.[7] It is considered more environmentally friendly than phosphates, which are more commonly encountered in detergent formulations.[7][8]
Dietary Supplement
This section needs more medical references for verification or relies too heavily on primary sources. (August 2025) |
Saccharic acid salts have found use in dietary supplements, where they act as precursors to the β-glucuronidase inhibitor saccharolactone (d-glucaro-1,4-lactone).[4] Some preclinical studies have demonstrated saccharolactone to have anticancerogenic and detoxification properties, although more clinical research is needed to confirm health impacts in humans.[9][10][11]
See also
- Saccharide
- Disaccharides
- Monosaccharides
- Mucic acid
- Gluconic acid
- Isosaccharinic acid
References
- ↑ "Human Metabolome Database: Showing metabocard for Glucaric acid (HMDB0000663)". https://www.hmdb.ca/metabolites/HMDB0000663#physical_properties.
- ↑ 2.0 2.1 PubChem. "Glucaric Acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/33037.
- ↑ Shendurse, A.M.; Khedkar, C.D. (2016). "Glucose: Properties and Analysis". Encyclopedia of Food and Health. Elsevier. pp. 239–247. doi:10.1016/b978-0-12-384947-2.00353-6. ISBN 978-0-12-384953-3. https://linkinghub.elsevier.com/retrieve/pii/B9780123849472003536. Retrieved 2025-08-10.
- ↑ 4.0 4.1 Shendurse, A. M.; Khedkar, C. D. (2016-01-01), Caballero, Benjamin; Finglas, Paul M.; Toldrá, Fidel, eds., Glucose: Properties and Analysis, Oxford: Academic Press, pp. 239–247, ISBN 978-0-12-384953-3, https://www.sciencedirect.com/science/article/pii/B9780123849472003536, retrieved 2025-06-17
- ↑ "Medical Definition of SACCHARIC ACID" (in en). https://www.merriam-webster.com/medical/saccharic+acid.
- ↑ 6.0 6.1 BeMiller, James N. (2019-01-01), BeMiller, James N., ed., "2 - Carbohydrate Reactions", Carbohydrate Chemistry for Food Scientists (Third Edition) (AACC International Press): pp. 25–48, ISBN 978-0-12-812069-9, https://www.sciencedirect.com/science/article/pii/B9780128120699000029, retrieved 2025-06-17
- ↑ 7.0 7.1 7.2 "D-Glucaric acid" (in en). https://www.acs.org/molecule-of-the-week/archive/g/glucaric-acid.html.
- ↑ Kogawa, Ana Carolina; Cernic, Beatriz Gamberini; do Couto, Leandro Giovanni Domingos; Salgado, Hérida Regina Nunes (February 2017). "Synthetic detergents: 100 years of history". Saudi Pharmaceutical Journal 25 (6): 934–938. doi:10.1016/j.jsps.2017.02.006. PMID 28951681.
- ↑ Saluk-Juszczak, Joanna; Olas, Beata; Nowak, Paweł; Staroń, Agnieszka; Wachowicz, Barbara (2008-07-01). "Protective effects of d-glucaro-1,4-lactone against oxidative modifications in blood platelets". Nutrition, Metabolism and Cardiovascular Diseases 18 (6): 422–428. doi:10.1016/j.numecd.2007.02.016. ISSN 0939-4753. PMID 17933501. https://www.sciencedirect.com/science/article/pii/S0939475307000890.
- ↑ Walaszek, Z. (1990-10-08). "Potential use of d-glucaric acid derivatives in cancer prevention". Cancer Letters 54 (1): 1–8. doi:10.1016/0304-3835(90)90083-A. ISSN 0304-3835. PMID 2208084. https://dx.doi.org/10.1016/0304-3835%2890%2990083-A.
- ↑ Ayyadurai, V. A. Shiva; Deonikar, Prabhakar; Fields, Christine (2023-02-01). "Mechanistic Understanding of D-Glucaric Acid to Support Liver Detoxification Essential to Muscle Health Using a Computational Systems Biology Approach". Nutrients 15 (3): 733. doi:10.3390/nu15030733. ISSN 2072-6643. PMID 36771439.
 |

