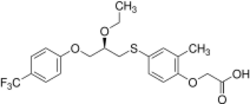
Chemistry:Seladelpar
 | |
| Clinical data | |
|---|---|
| Other names | MBX-8025; RWJ-800025 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H23F3O5S |
| Molar mass | 444.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Seladelpar (INN, USAN; developmental code names MBX-8025, RWJ-800025) is a PPARδ receptor agonist that is being investigated for drug use by Metabolex.[1][2][3] According to a press release they are examining its potential use for the treatment of dyslipidemia, metabolic syndrome, type 2 diabetes, and non-alcoholic steatohepatitis (NASH). The compound was licensed from Janssen Pharmaceutica NV.[4] The drug completed a phase II trial for primary biliary cholangitis. "Seladelpar demonstrated robust, dose-dependent, clinically significant, and durable improvements in biochemical markers of cholestasis and inflammation in patients with PBC at risk of disease progression. Seladelpar appeared safe and well tolerated and was not associated with any increase in pruritus."[5] A phase III trial in patients with PBC also found reduced pruritus and improved liver biochemistry, despite being terminated early.[6]
See also
References
- ↑ "Seladelpar - CymaBay Therapeutics". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800024671.
- ↑ "PPAR-beta/delta agonists for Type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home". Expert Opinion on Investigational Drugs 17 (10): 1465–1471. October 2008. doi:10.1517/13543784.17.10.1465. PMID 18808307.
- ↑ "MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin". The Journal of Clinical Endocrinology and Metabolism 96 (9): 2889–2897. September 2011. doi:10.1210/jc.2011-1061. PMID 21752880.
- ↑ "Targeting Mixed Dyslipidemia and Metabolic Syndrome". Metabolex, Inc.. 2005. http://www.metabolex.com/MBX-8025.html.
- ↑ "A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis". Journal of Hepatology 77 (2): 353–364. August 2022. doi:10.1016/j.jhep.2022.02.033. PMID 35367282. https://eprints.ncl.ac.uk/fulltext.aspx?url=281880/6A8E4677-1BB4-456F-AFB5-1B8D7DEE7B93.pdf&pub_id=281880.
- ↑ "Seladelpar efficacy and safety at 3 months in patients with primary biliary cholangitis: ENHANCE, a phase 3, randomized, placebo-controlled study". Hepatology 78 (2): 397–415. August 2023. doi:10.1097/HEP.0000000000000395. PMID 37386786.
External links
 |

