| Display title | Chemistry:Imide |
| Default sort key | Imide |
| Page length (in bytes) | 9,145 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 803046 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 0 |
| Counted as a content page | Yes |
| Page image | 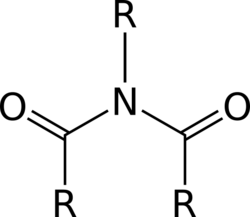 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>Dennis Ross |
| Date of page creation | 11:16, 9 September 2023 |
| Latest editor | imported>Dennis Ross |
| Date of latest edit | 11:16, 9 September 2023 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength... |

