| Display title | Chemistry:Platinum disulfide |
| Default sort key | Platinum disulfide |
| Page length (in bytes) | 1,859 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 816668 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 1 |
| Counted as a content page | Yes |
| Page image | 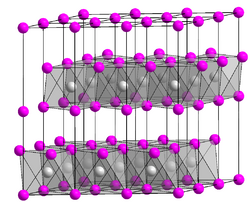 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>Rtextdoc |
| Date of page creation | 19:27, 5 February 2024 |
| Latest editor | imported>Rtextdoc |
| Date of latest edit | 19:27, 5 February 2024 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | Platinum disulfide is the inorganic compound with the formula PtS2. It is a black, semiconducting solid, which is insoluble in all solvents. The compound adopts the cadmium iodide structure, being composed of sheets of octahedral Pt and pyramidal sulfide centers. Single crystals are grown by chemical... |

