| Display title | Chemistry:Thionyl |
| Default sort key | Thionyl |
| Page length (in bytes) | 855 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 818162 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 0 |
| Counted as a content page | Yes |
| Page image | 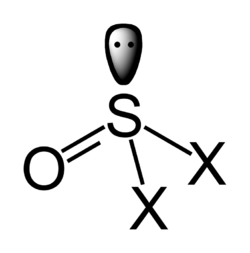 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>NBrush |
| Date of page creation | 02:37, 30 June 2021 |
| Latest editor | imported>NBrush |
| Date of latest edit | 02:37, 30 June 2021 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | The thionyl group is SO, a sulfur atom plus an oxygen atom.
It occurs in compounds such as thionyl fluoride, SOF2.
Thionyl chloride, SOCl2, is a common reagent used in organic synthesis to convert carboxylic acids to acyl chlorides.
In organic chemistry, the thionyl group is known as a sulfoxide group... |

