| Display title | Chemistry:Thiuram disulfide |
| Default sort key | Thiuram disulfide |
| Page length (in bytes) | 4,627 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 24705 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 0 |
| Counted as a content page | Yes |
| Page image | 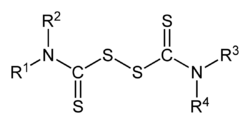 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>Unex |
| Date of page creation | 19:17, 5 February 2024 |
| Latest editor | imported>Unex |
| Date of latest edit | 19:17, 5 February 2024 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as in the manufacture... |

