| Display title | Chemistry:Triplet oxygen |
| Default sort key | Triplet oxygen |
| Page length (in bytes) | 9,363 |
| Namespace ID | 3022 |
| Namespace | Chemistry |
| Page ID | 436691 |
| Page content language | en - English |
| Page content model | wikitext |
| Indexing by robots | Allowed |
| Number of redirects to this page | 0 |
| Counted as a content page | Yes |
| Page image | 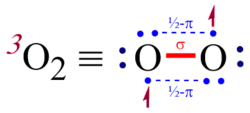 |
| HandWiki item ID | None |
| Edit | Allow all users (infinite) |
| Move | Allow all users (infinite) |
| Page creator | imported>NBrushPhys |
| Date of page creation | 06:26, 28 June 2021 |
| Latest editor | imported>NBrushPhys |
| Date of latest edit | 06:26, 28 June 2021 |
| Total number of edits | 1 |
| Recent number of edits (within past 90 days) | 0 |
| Recent number of distinct authors | 0 |
Description | Content |
Article description: (description)
This attribute controls the content of the description and og:description elements. | Triplet oxygen, 3O2, refers to the S = 1 electronic ground state of molecular oxygen (dioxygen). It is the most stable and common allotrope of oxygen. Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unusual example of a stable and commonly encountered diradical... |
