Chemistry:Hydroxyquinone
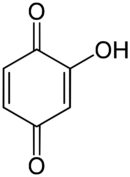
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C6H4O3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by an hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone (which often called just "quinone").
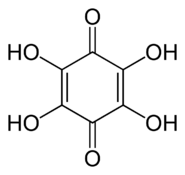
More generally, the term may refer to any derivative of any quinone (such as 1,2-benzoquinone, 1,4-naphthoquinone or 9,10-anthraquinone), where any number n of hydrogens have been replaced by n hydroxyls. In this case the number n is indicated by a multiplier prefix (mono-, di-, tri-, etc.), and the parent quinone's name is used instead of just "quinone" — as in tetrahydroxy-1,4-benzoquinone.
The hydroxyquinones (in the particular or the general sense) include many biologically and industrially important compounds, and are a building block of many medicinal drugs.
Hydroxyquinones with hydroxyls adjacent to the ketone groups often exhibit intramolecular hydrogen bonding, which affects their redox properties and their biochemical properties.[1]
The term "hydroxyquinone" should not be confused with hydroquinone, the common name of benzene-1,4-diol.
Subfamilies
- Hydroxybenzoquinones
- Hydroxynaphthoquinones
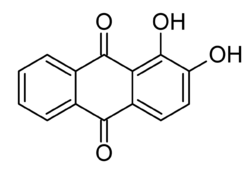
- Hydroxyanthraquinones
References
- ↑ J. Khalafy and J.M. Bruce (2002), Oxidative dehydrogenation of 1-tetralones: Synthesis of juglone, naphthazarin, and [alpha]-hydroxyanthraquinones. Journal of Sciences, Islamic Republic of Iran, volume 13 issue 2, pages 131-139.