Chemistry:Corey–Nicolaou macrolactonization
From HandWiki
| Corey–Nicolaou macrolactonization | |
|---|---|
| Named after | Elias James Corey K. C. Nicolaou |
| Reaction type | Name reaction |
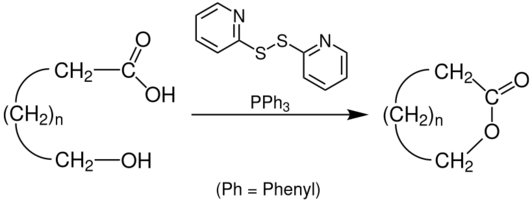
Corey–Nicolaou macrolactonization is a named reaction of organic chemistry, for the synthesis of lactones from hydroxy acids, found in 1974.[1][2] The reaction should take place in a polar aprotic solvent with mild conditions,[3] with the use of 2,2'-Dipyridyldisulfide and triphenylphosphine.
Mechanism
The hydroxy acid first reacts with the 2,2'-Dipyridyldisulfide to form the corresponding 2-pyridinethiol ester, and after a proton transfer, the alkoxide attacks the carbonyl carbon, forming a tetrahedral transition state, before resolving back to the desired lactone and 2-pyridinethione.[4][5]
See also
References
- ↑ Corey, Elias James; Nicolaou, Kyriacos Costa (1974-08-01). "Efficient and mild lactonization method for the synthesis of macrolides.". Journal of the American Chemical Society 96 (17): 5614–5616. doi:10.1021/ja00824a073.
- ↑ Corey, Elias James; Nicolaou, Kyriacos Costa; Melvin, Lawrence S., Jr. (1975). "Synthesis of novel macrocyclic lactones in the prostaglandin and polyether antibiotic series". Journal of the American Chemical Society 97 (3): 653–654. doi:10.1021/ja00836a036. PMID 1133366.
- ↑ Meng, Qingchang; Hesse, Manfred (1992). "Ring-closure methods in the synthesis of macrocyclic natural products". Topics in Current Chemistry 161: 107–176. doi:10.1007/3-540-54348-1_9. ISBN 978-3-540-54348-0.
- ↑ Corey, Elias James; Brunelle, Daniel J.; Stork, Philip J. (1976). "Mechanistic studies on the double activation method for the synthesis of macrocyclic lactones". Tetrahedron Letters 17 (38): 3405–3408. doi:10.1016/S0040-4039(00)93056-9.
- ↑ Behinpour, Kevian; Hopkins, Andrew; Williiams, Andrew (1981). "Macrolide ring closuke: "Double activation" mechanism". Tetrahedron Letters 22 (4): 275–278. doi:10.1016/0040-4039(81)80074-3.