Chemistry:NacNac
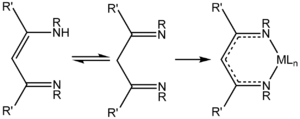
NacNac is a class of anionic bidentate ligands. 1,3-Diketimines are often referred to as "HNacNac", a modification of the abbreviation Hacac used for 1,3-diketones. These species can exist as a mixture of tautomers.[1]
Preparation of ligands and complexes
Acetylacetone and related 1,3-diketones condense with primary alkyl- or arylamines resulting in replacement of the carbonyl oxygen atoms with NR groups, where R = aryl, alkyl. To prepare 1,3-diketimines from bulky amines, e.g. 2,4,6-trimethylanilines, prolonged reaction times are required. 2,6-Diisopropylaniline is a common bulky building block.
Deprotonation of HNacNac compounds affords anionic bidentate ligands that form a variety of coordination complexes.[2] Some derivatives with large R groups can be used to stabilize low valent main group and transition metal complexes.[3] Unlike the situation for the acetylacetonates, the steric properties of the coordinating atoms in NacNac− ligands is adjustable by changes in the R substituent. Attachment to a metal center is usually carried out by initial deprotonation of HNacNac with n-butyllithium; the lithium derivative is then treated with a metal chloride to eliminate lithium chloride. In some cases, HNacNacs also serve as charge-neutral 1,3-diimine ligands.

Related NacNac ligands

NacNac ligands are diimine analogues of acetylacetonate ligands. An intermediate class of ligands are derived from monoimino-ketones.[5][6] The first Dipp-NacNac ligand was synthesized by Dr. Francis S. Mair in 1998.[7]
See also
References
- ↑ Mindiola, D. J.; Holland, P. L.; Warren, T. H. (2010). "Complexes of Bulky β-Diketiminate Ligands". Inorganic Syntheses 35: 1–55. doi:10.1002/9780470651568.ch1.
- ↑ Bourget-Merle, L.; Lappert, M. F.; Severn, J. R. (2002). "The Chemistry of Diketiminatometal Complexes". Chemical Reviews 102: 3031–3066. doi:10.1021/cr010424r.
- ↑ Qian, B.; Ward, D. L.; Smith, M.R. (1998). "Synthesis, Structure, and Reactivity of β-Diketiminato Aluminum Complexes". Organometallics 17: 3070–3076. doi:10.1021/om970886o.
- ↑ Hartmann, N.J.; Wu, G.; Hayton, T. W. (2015). "Synthesis of a "Masked" Terminal Nickel(II) Sulfide by Reductive Deprotection and its Reaction with Nitrous Oxide". Angew. Chem. Int. Ed. Engl. 54: 14956-9. doi:10.1002/anie.201508232. PMID 26457792.
- ↑ 5.0 5.1 Weber, Birgit; Jäger, Ernst-G. (2009). "Structure and Magnetic Properties of Iron(II/III) Complexes with N2O2–2-Coordinating Schiff Base-Like Ligands". Eur. J. Inorg. Chem. 2009: 455. doi:10.1002/ejic.200990003.
- ↑ Riley, Dennis P.; Busch, Daryle H. (1978). "Macrocyclic Tetraazatetraenato Ligands and their Metal Complexes". Inorg. Synth. 18: 36. doi:10.1002/9780470132494.ch7.
- ↑ Mair, Frank S.; Cope, Elaine K.; Clegg, William; Edwards, Andrew J. (1998). "Structural Characterization of [(2,6-Pri2C6H3)NC(Me)C(H)C(Me)N(2,6-Pri2C6H3)K·PhCH3]∞: A Heavy Alkali Metal Diazapentadienyl Complex". Inorg. Chem. 37. doi:10.1021/ic970956j.
 |

