Physics:Probe electrospray ionization
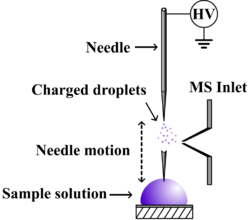
Probe electrospray ionization (PESI) is an electrospray-based ambient ionization technique which is coupled with mass spectrometry for sample analysis.[1][2] Unlike traditional mass spectrometry ion sources which must be maintained in a vacuum, ambient ionization techniques permit sample ionization under ambient conditions, allowing for the high-throughput analysis of samples in their native state, often with minimal or no sample pre-treatment.[3] The PESI ion source simply consists of a needle to which a high voltage is applied following sample pick-up, initiating electrospray directly from the solid needle.
History
Probe electrospray ionization is an ambient ionization mass spectrometry technique developed by Kenzo Hiraoka et al. at the University of Yamanashi, Japan.[4] The technique was developed to address some of the issues associated with traditional electrospray ionization (ESI), including clogging of the capillary and contamination, whilst providing a means of rapid and direct sample analysis. Since its initial conception, various modified forms of the PESI ion source have been developed, and the PESI-MS system has been commercialized by instrument manufacturing company Shimadzu.
Principle of operation
The PESI ion source consists of a solid needle or wire which acts as both the sampling probe and electrospray emitter.[5] The needle is moved up and down along a vertical axis, a process which can be either automated or manual. When the needle is lowered to the sampling stage, the tip of the needle briefly touches the surface of a typical liquid sample. During this stage, the needle is held at ground potential. The needle is then raised to be level with the mass spectrometer inlet where a high voltage of 2–3 kV is applied. Electrospray is induced at the tip of the needle, producing analyte ions which are drawn into the mass spectrometer for analysis. The mechanism by which ions are formed is believed to be identical to traditional electrospray ionization. As a result, in positive ion mode analytes are often observed as the protonated, sodiated and potentiated ions, depending on the sample and analyte type.
Although the amount of sample picked up by the needle is largely dependent on sample viscosity, it has been estimated that just a few picolitres of the sample solution are typically used.[6]
Because of this, the technique can be applied to small sample sizes, particularly ideal when limited sample amounts are available. As such a small sample amount is picked up and completely exhausted during the ionization process, issues of contamination are severely reduced. Furthermore, the process of sampling and ionization takes just a few seconds, so PESI-MS is suitable for high-throughput analysis.
Sequential ionization
A phenomenon observed with probe electrospray ionization is the sequential and exhaustive ionization of analytes with different surface activities. During the development of PESI, it was discovered that analytes could be sequentially ionized throughout the electrospray, thus enabling a temporal separation of components within a sample.[7] In normal ESI, the sample solution is typically continuously supplied through a capillary and the charged droplets contain all sample components, with more surface-active analytes being constantly preferentially ionized. In PESI, surface-active analytes are also preferentially ionized. However, as a finite droplet exists on the tip of the needle, following the depletion of surface-active analytes, the remaining components in the droplet can then be ionized and observed. This can result in the production of distinctively different mass spectra from a single sample over the application of the high voltage for just a few seconds.
This effect offers a particular advantage in the analysis of analytes suffering from ion suppression effects. The presence of surface-active analytes or charged solvent additives can result in the suppressed ionization of analytes of interest, resulting in low sensitivity or the complete absence of the analyte.[7] The effects of ion suppression can be minimized by reducing the complexity of the sample, for instance through sample extraction techniques such as solid phase extraction, or by separation of analytes of interest using chromatographic separation. However, these sample preparation steps can be laborious, time-consuming and expensive. PESI enables a reduction in ion suppression without the need for sample pre-treatment. By separating the ionization of different analytes, components causing ion suppression can be exhausted before enabling the ionization of components of interest. This has been demonstrated in a number of scenarios, including in the analysis of raw urine, with concentrated components such as creatinine ionization initially, followed by the appearance of previously undetected metabolites.[8]
Sheath-flow PESI
As the PESI needle is only applicable to liquid or penetrable solid samples, it cannot be used for the analysis of the majority of dry solid materials. To circumvent this limitation, sheath-flow probe electrospray ionization (sfPESI) was developed, a modification of the traditional PESI technique. The sfPESI ion source consists of a solid needle housed within a plastic sheath (typically a gel-loading tip) filled with a small amount of solvent. The needle protrudes from the base of the sheath by approximately 0.1 mm, where a minute solvent droplet is held. The based of[clarification needed] based the probe is briefly touched to the sample surface, where a convex solvent meniscus forms between the probe and the sample, wetting the sample and enabling analyte extraction.[9] The chemistry of the solvent can be modified to induce the extraction of particular analytes of interest. After application to the sample, the sfPESI probe is then raised to be level with the mass spectrometer inlet, with solubilised analytes held in the droplet at the tip of the needle, and a high voltage applied. sfPESI offers the same advantages as standard PESI, including the sequential and exhaustive ionization phenomenon, whilst enabling the direct analysis of dry samples.
Applications
PESI-MS has proven to be particularly effective in the metabolic analysis of biological materials, having been applied to the analysis of cancerous and non-cancerous breast tissue,[10] as well as brain and liver tissue removed from mice.[11][12] Interestingly, PESI-MS has recently been applied to the direct analysis of living animals for real-time metabolic profiling.[13][14] Due to the narrow diameter of the PESI needle and brief sample introduction time, PESI is reasonably non-invasive. As a result, the technique has been used to sample from the organs of living anaesthetized animals, specifically to analyse metabolites in the brain, spleen, liver and kidney of a living mouse. In addition to this, PESI-MS has been applied to the on-site analysis of food products for the purpose of quality control, to the detection of herbicides in body fluids to demonstrate exposure, and finally to the detection of illicit drugs in bodily fluids to indicate drug use. Several groups have also harnessed the small size of the PESI probe to achieve single-cell analysis, demonstrating the capability of rapidly detecting metabolites at cellular and subcellular levels.[15][16][17]
The PESI modification known as sheath-flow PESI has been applied to the analysis of various solid samples in their native state, including pharmaceutical tablets,[9] illicit drugs,[5] food and agricultural products,[18] and pesticides.[19] In addition, sfPESI has been utilised in the field of forensic science for the analysis and identification of fresh and dried body fluids of forensic interest.[8] In this work, sfPESI was also coupled with tandem mass spectrometry (MS/MS), demonstrating the capability of ion fragmentation for identification of unknown components.
See also
References
- ↑ Hiraoka, Kenzo; Usmanov, Dilshadbek T.; Chen, Lee Chuin; Ninomiya, Satoshi; Mandal, Mridul K.; Saha, Subhrakanti (2015). "Probe electrospray ionization (PESI) mass spectrometry with discontinuous atmospheric pressure interface (DAPI)". European Journal of Mass Spectrometry 21 (3): 327–334. doi:10.1255/ejms.1309. PMID 26307713.
- ↑ Usmanov, Dilshadbek T.; Mandal, Mridul K.; Hiraoka, Kenzo; Ninomiya, Satoshi; Wada, Hiroshi; Matsumura, Masaya; Sanada-Morimura, Sachiyo; Nonami, Hiroshi et al. (2018-09-15). "Dipping probe electrospray ionization/mass spectrometry for direct on-site and low-invasive food analysis". Food Chemistry 260: 53–60. doi:10.1016/j.foodchem.2018.04.003. PMID 29699681.
- ↑ Cooks, R. G. (2006-03-17). "Ambient Mass Spectrometry". Science 311 (5767): 1566–1570. doi:10.1126/science.1119426. ISSN 0036-8075. PMID 16543450. Bibcode: 2006Sci...311.1566C.
- ↑ Hiraoka, Kenzo; Nishidate, Kentaro; Mori, Kunihiko; Asakawa, Daiki; Suzuki, Shigeo (2007-09-30). "Development of probe electrospray using a solid needle". Rapid Communications in Mass Spectrometry 21 (18): 3139–3144. doi:10.1002/rcm.3201. PMID 17708527. Bibcode: 2007RCMS...21.3139H.
- ↑ 5.0 5.1 Rahman, Md. Obaidur; Mandal, Mridul Kanti; Shida, Yasuo; Ninomiya, Satoshi; Chen, Lee Chuin; Nonami, Hiroshi; Hiraoka, Kenzo (July 2013). "Development of sheath-flow probe electrospray ionization (SF-PESI): Sheath-flow probe electrospray ionization/SF-PESI". Journal of Mass Spectrometry 48 (7): 823–829. doi:10.1002/jms.3226. PMID 23832938.
- ↑ Yoshimura, Kentaro; Chen, Lee Chuin; Asakawa, Daiki; Hiraoka, Kenzo; Takeda, Sen (June 2009). "Physical properties of the probe electrospray ionization (PESI) needle applied to the biological samples". Journal of Mass Spectrometry (United States Department of Agriculture) 44 (6): 978–985. doi:10.1002/jms.1576. PMID 19306264. Bibcode: 2009JMSp...44..978Y. https://pubag.nal.usda.gov/catalog/2182920.
- ↑ 7.0 7.1 Mandal, Mridul Kanti; Chen, Lee Chuin; Hiraoka, Kenzo (September 2011). "Sequential and Exhaustive Ionization of Analytes with Different Surface Activity by Probe Electrospray Ionization". Journal of the American Society for Mass Spectrometry 22 (9): 1493–1500. doi:10.1007/s13361-011-0162-4. ISSN 1044-0305. PMID 21953252. Bibcode: 2011JASMS..22.1493M.
- ↑ 8.0 8.1 Rankin-Turner, Stephanie; Ninomiya, Satoshi; Reynolds, James C.; Hiraoka, Kenzo (2019). "Sheath-flow probe electrospray ionization (sfPESI) mass spectrometry for the rapid forensic analysis of human body fluids". Analytical Methods 11 (29): 3633–3640. doi:10.1039/C9AY00698B. ISSN 1759-9660.
- ↑ 9.0 9.1 Usmanov, Dilshadbek T.; Ashurov, Khatam B.; Ninomiya, Satoshi; Hiraoka, Kenzo; Wada, Hiroshi; Nakano, Hiroshi; Matsumura, Masaya; Sanada-Morimura, Sachiyo et al. (2018-03-15). "Remote sampling mass spectrometry for dry samples: Sheath-flow probe electrospray ionization (PESI) using a gel-loading tip inserted with an acupuncture needle". Rapid Communications in Mass Spectrometry 32 (5): 407–413. doi:10.1002/rcm.8045. PMID 29235697. Bibcode: 2018RCMS...32..407U.
- ↑ Mandal, Mridul Kanti; Yoshimura, Kentaro; Chen, Lee Chuin; Yu, Zhan; Nakazawa, Tadao; Katoh, Ryohei; Fujii, Hideki; Takeda, Sen et al. (November 2012). "Application of Probe Electrospray Ionization Mass Spectrometry (PESI-MS) to Clinical Diagnosis: Solvent Effect on Lipid Analysis". Journal of the American Society for Mass Spectrometry 23 (11): 2043–2047. doi:10.1007/s13361-012-0462-3. ISSN 1044-0305. PMID 22923015. Bibcode: 2012JASMS..23.2043M.
- ↑ Hayashi, Yumi; Zaitsu, Kei; Murata, Tasuku; Ohara, Tomomi; Moreau, Stéphane; Kusano, Maiko; Tanihata, Hiroshi; Tsuchihashi, Hitoshi et al. (August 2017). "Intact metabolite profiling of mouse brain by probe electrospray ionization/triple quadrupole tandem mass spectrometry (PESI/MS/MS) and its potential use for local distribution analysis of the brain". Analytica Chimica Acta 983: 160–165. doi:10.1016/j.aca.2017.06.047. PMID 28811022.
- ↑ Zaitsu, Kei; Hayashi, Yumi; Murata, Tasuku; Ohara, Tomomi; Nakagiri, Kenta; Kusano, Maiko; Nakajima, Hiroki; Nakajima, Tamie et al. (2016-04-05). "Intact Endogenous Metabolite Analysis of Mice Liver by Probe Electrospray Ionization/Triple Quadrupole Tandem Mass Spectrometry and Its Preliminary Application to in Vivo Real-Time Analysis". Analytical Chemistry 88 (7): 3556–3561. doi:10.1021/acs.analchem.5b04046. ISSN 0003-2700. PMID 26958983.
- ↑ Yoshimura, Kentaro; Chen, Lee Chuin; Johno, Hisashi; Nakajima, Mayutaka; Hiraoka, Kenzo; Takeda, Sen (2015). "Development of Non-proximate Probe Electrospray Ionization for Real-Time Analysis of Living Animal". Mass Spectrometry 3 (Special_Issue_3): S0048. doi:10.5702/massspectrometry.S0048. ISSN 2186-5116. PMID 26819892.
- ↑ Zaitsu, Kei; Hayashi, Yumi; Murata, Tasuku; Yokota, Kazumi; Ohara, Tomomi; Kusano, Maiko; Tsuchihashi, Hitoshi; Ishikawa, Tetsuya et al. (2018-04-03). "In Vivo Real-Time Monitoring System Using Probe Electrospray Ionization/Tandem Mass Spectrometry for Metabolites in Mouse Brain". Analytical Chemistry 90 (7): 4695–4701. doi:10.1021/acs.analchem.7b05291. ISSN 0003-2700. PMID 29519127.
- ↑ Gong, Xiaoyun; Zhao, Yaoyao; Cai, Shaoqing; Fu, Shujie; Yang, Chengdui; Zhang, Sichun; Zhang, Xinrong (2014-04-15). "Single Cell Analysis with Probe ESI-Mass Spectrometry: Detection of Metabolites at Cellular and Subcellular Levels". Analytical Chemistry 86 (8): 3809–3816. doi:10.1021/ac500882e. ISSN 0003-2700. PMID 24641101.
- ↑ Chen, Fengming; Lin, Luyao; Zhang, Jie; He, Ziyi; Uchiyama, Katsumi; Lin, Jin-Ming (2016-04-19). "Single-Cell Analysis Using Drop-on-Demand Inkjet Printing and Probe Electrospray Ionization Mass Spectrometry". Analytical Chemistry 88 (8): 4354–4360. doi:10.1021/acs.analchem.5b04749. ISSN 0003-2700. PMID 27015013.
- ↑ Nakashima, Taiken; Wada, Hiroshi; Morita, Satoshi; Erra-Balsells, Rosa; Hiraoka, Kenzo; Nonami, Hiroshi (2016-03-15). "Single-Cell Metabolite Profiling of Stalk and Glandular Cells of Intact Trichomes with Internal Electrode Capillary Pressure Probe Electrospray Ionization Mass Spectrometry". Analytical Chemistry 88 (6): 3049–3057. doi:10.1021/acs.analchem.5b03366. ISSN 0003-2700. PMID 26845634.
- ↑ Hiraoka, Kenzo; Rankin-Turner, Stephanie; Ninomiya, Satoshi; Wada, Hiroshi; Nakano, Hiroshi; Matsumura, Masaya; Sanada-Morimura, Sachiyo; Tanaka, Fukuyo et al. (2019-03-20). "Component Profiling in Agricultural Applications Using an Adjustable Acupuncture Needle for Sheath-Flow Probe Electrospray Ionization/Mass Spectrometry". Journal of Agricultural and Food Chemistry 67 (11): 3275–3283. doi:10.1021/acs.jafc.8b06424. ISSN 0021-8561. PMID 30830775.
- ↑ Mandal, Mridul Kanti; Ozawa, Tomoyuki; Saha, Subhrakanti; Rahman, Md. Matiur; Iwasa, Mami; Shida, Yasuo; Nonami, Hiroshi; Hiraoka, Kenzo (2013-08-21). "Development of Sheath-Flow Probe Electrospray Ionization Mass Spectrometry and Its Application to Real Time Pesticide Analysis". Journal of Agricultural and Food Chemistry 61 (33): 7889–7895. doi:10.1021/jf4014718. ISSN 0021-8561. PMID 23875640.
 |