Earth:Bioassay
A bioassay is an analytical method to determine the potency or effect of a substance by its effect on living animals or plants (in vivo), or on living cells or tissues (in vitro).[1][2] A bioassay can be either quantal or quantitative, direct or indirect.[3] If the measured response is binary, the assay is quantal; if not, it is quantitative.[3]
A bioassay may be used to detect biological hazards or to give an assessment of the quality of a mixture.[4] A bioassay is often used to monitor water quality as well as wastewater discharges and its impact on the surroundings.[5] It is also used to assess the environmental impact and safety of new technologies and facilities.[citation needed]
Principle
A bioassay is a biochemical test to estimate the potency of a sample compound. Usually this potency can only be measured relative to a standard compound.[3][1] A typical bioassay involves a stimulus (ex. drugs) applied to a subject (ex. animals, tissues, plants). The corresponding response (ex. death) of the subject is thereby triggered and measured.[6]
History
The first use of a bioassay dates back to the late 19th century, when the foundation of bioassays was laid down by German physician Paul Ehrlich.[7] He introduced the concept of standardization by the reactions of living matter.[7][6] His bioassay on diphtheria antitoxin was the first bioassay to receive recognition.[8] His use of bioassay was able to discover that administration of gradually increasing dose of diphtheria in animals stimulated production of antiserum.[9]
One well known example of a bioassay is the "canary in the coal mine" experiment.[10] To provide advance warning of dangerous levels of methane in the air, miners would take methane-sensitive canaries into coal mines. If the canary died due to a build-up of methane, the miners would leave the area as quickly as possible.
Many early examples of bioassays used animals to test the carcinogenicity of chemicals.[11] In 1915, Yamaigiwa Katsusaburo and Koichi Ichikawa tested the carcinogenicity of coal tar using the inner surface of rabbit's ears.[11]
From the 1940s to the 1960s, animal bioassays were primarily used to test the toxicity and safety of drugs, food additives, and pesticides.[11]
Beginning in the late 1960s and 1970s, reliance on bioassays increased as public concern for occupational and environmental hazards increased.[11]
Classifications
Bioassay can be classified by how it is applied and how the response is recorded.
- Direct assay
- In a direct assay, the stimulus applied to the subject is specific and directly measurable, and the response to that stimulus is recorded. The variable of interest is the specific stimulus required to produce a response of interest (ex. death of the subject).[6][12]
- Indirect assay
- In an indirect assay, the stimulus is fixed in advance and the response is measured in the subjects. The variable of interest in the experiment is the response to a fixed stimulus of interest.[6][12]
- Quantitative response
- The measurement of the response to the stimulus is on a continuous scale (ex. blood sugar content, degree of color change in cell growth medium).[12]
- Quantal response
- The response is binary; it is a determination of whether or not an event occurs (ex. death of the subject).[12]
Examples
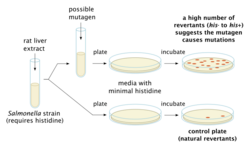
One classical bioassay is the Ames test. A strain of Salmonella that requires histidine to grow is put on two plates with growth medium containing minimal amounts of histidine and some rat liver extract (to mimick liver metabolism). A suspected mutagen is added to one plate. If the plate with the suspected mutagen grows more visible colonies, it is probably mutagenic: a mutagen might cause the strain of bacterium to regain the ability to make its own histidine.[13]
Most other forms of toxicology testing are also bioassays. Animals or cell cultures may be put under a number of levels of a suspected toxin to ascertain whether the substance causes harmful changes and at what level it does so. The -1">50 value, a common measure of acute toxicity, describes the dose at which a substance is lethal to 50% of tested animals.[14]
The potency of a drug may be measured using a bioassay.[15]
Environmental bioassays
Environmental bioassays are generally a broad-range survey of toxicity.[16] A toxicity identification evaluation is conducted to determine what the relevant toxicants are. Although bioassays are beneficial in determining the biological activity within an organism, they can often be time-consuming and laborious. Organism-specific factors may result in data that are not applicable to others in that species. For these reasons, other biological techniques are often employed, including radioimmunoassays. See bioindicator.
Water pollution control requirements in the United States require some industrial dischargers and municipal sewage treatment plants to conduct bioassays. These procedures, called whole effluent toxicity tests, include acute toxicity tests as well as chronic test methods.[5] The methods involve exposing living aquatic organisms to samples of wastewater for a specific length of time.[17][18] Another example is the bioassay ECOTOX, which uses the microalgae Euglena gracilis to test the toxicity of water samples.[19] (See Bioindicator)
See also
- Assay
- Immunoassay
- Umu Chromotest
References
- ↑ 1.0 1.1 Hoskins, W. M.; Craig, R. (1962-01-01). "Uses of Bioassay in Entomology". Annual Review of Entomology 7 (1): 437–464. doi:10.1146/annurev.en.07.010162.002253. ISSN 0066-4170. PMID 14449182.
- ↑ "Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products". Washington, D.C.: U.S. Food and Drug Administration. January 2011. p. 7. https://www.fda.gov/media/79856/download.
- ↑ 3.0 3.1 3.2 Laska, E M; Meisner, M J (1987-04-01). "Statistical Methods and Applications of Bioassay". Annual Review of Pharmacology and Toxicology 27 (1): 385–397. doi:10.1146/annurev.pa.27.040187.002125. ISSN 0362-1642. PMID 3579242.
- ↑ Prinsloo, Gerhard; Papadi, Georgia; Hiben, Mebrahtom G.; Haan, Laura de; Louisse, Jochem; Beekmann, Karsten; Vervoort, Jacques; Rietjens, Ivonne M.C.M. (2017). "In vitro bioassays to evaluate beneficial and adverse health effects of botanicals: promises and pitfalls". Drug Discovery Today 22 (8): 1187–1200. doi:10.1016/j.drudis.2017.05.002. PMID 28533190.
- ↑ 5.0 5.1 "Permit Limits-Whole Effluent Toxicity (WET)". Washington, D.C.: U.S. Environmental Protection Agency (EPA). 2021-10-11. https://www.epa.gov/npdes/permit-limits-whole-effluent-toxicity-wet.
- ↑ 6.0 6.1 6.2 6.3 Saha, G. M (29 November 2002). Design and Analysis for Bioassays. Kolkata: Indian Statistical Institute. pp. 61–76.
- ↑ 7.0 7.1 Van Noordwijk, Jacobus (1989). "Bioassays in whole animals". Journal of Pharmaceutical and Biomedical Analysis 7 (2): 139–145. doi:10.1016/0731-7085(89)80077-9. PMID 2488614.
- ↑ Analysis of foods and beverages : modern techniques. Charalambous, George, 1922-1994.. Orlando: Academic Press. 1984. ISBN 0121691608. OCLC 9682930. https://archive.org/details/analysisoffoodsb0000unse.
- ↑ Bosch, Fèlix; Rosich, Laia (2008). "The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize" (in en). Pharmacology 82 (3): 171–179. doi:10.1159/000149583. ISSN 0031-7012. PMID 18679046.
- ↑ "Environmental Inquiry - How Are Bioassays Used in the Real World?". http://ei.cornell.edu/toxicology/bioassays/Uses.html.
- ↑ 11.0 11.1 11.2 11.3 Beyer, L. A .; Beck, B. D.; Lewandowski, T. A. (2011-04-01). "Historical perspective on the use of animal bioassays to predict carcinogenicity: Evolution in design and recognition of utility". Critical Reviews in Toxicology 41 (4): 321–338. doi:10.3109/10408444.2010.541222. ISSN 1040-8444. PMID 21438739.
- ↑ 12.0 12.1 12.2 12.3 Le, Chap. "Direct Bioassays & Estimation of Ratios". http://www.biostat.umn.edu/~chap/S06-DirectBioassay.pdf.
- ↑ Charnley G (2002). "Ames Test". Encyclopedia of Public Health. eNotes.com. http://www.enotes.com/public-health-encyclopedia/ames-test. Retrieved 2014-05-02.
- ↑ "Absolute lethal dose (LD100)" (in en). IUPAC Gold Book. International Union of Pure and Applied Chemistry. https://goldbook.iupac.org/html/A/A00025.html.
- ↑ Qin, X; Yao, W; Shi, X; Liu, L; Huang, F; Ding, Y; Zhou, Y; Yu, L et al. (7 March 2019). "Responsive Cells for rhEGF bioassay Obtained through Screening of a CRISPR/Cas9 Library.". Scientific Reports 9 (1): 3780. doi:10.1038/s41598-019-40381-4. PMID 30846752.
- ↑ "Cytotoxic Activity of Herbal Medicines as Assessed in Vitro: A Review". Chemistry & Biodiversity 20 (2): 3–27. January 2023. doi:10.1002/cbdv.202201098. PMID 36595710.
- ↑ Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (Report). EPA. October 2002. EPA-821-R-02-012. https://www.epa.gov/cwa-methods/acute-toxicity-wet-methods.
- ↑ "Whole Effluent Toxicity Methods". EPA. 2020-08-01. https://www.epa.gov/cwa-methods/whole-effluent-toxicity-methods.
- ↑ Tahedl, Harald; Hader, Donat-Peter (1999). "Fast examination of water quality using the automatic biotest ECOTOX based on the movement behavior of a freshwater flagellate". Water Research 33 (2): 426–432. doi:10.1016/s0043-1354(98)00224-3. Bibcode: 1999WatRe..33..426T.
 |