Chemistry:Chan rearrangement
From HandWiki
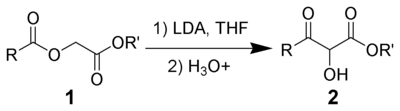
The Chan rearrangement is a chemical reaction that involves rearranging an acyloxy acetate (1) in the presence of a strong base to a 2-hydroxy-3-keto-ester (2).[1]
This procedure was employed in the Holton Taxol total synthesis.[2]
Reaction mechanism
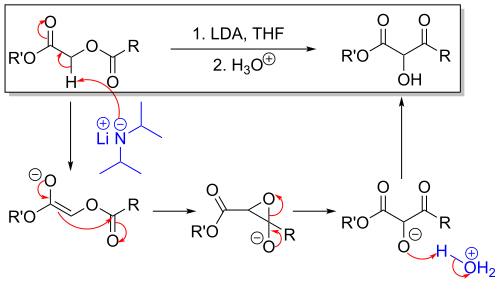
The methylene bridge in the reactant with adjacent carbonyl and acetyl substituents is acidic and can be deprotonated by strong non-nucleophilic bases such as lithium tetramethylpiperidide or lithium diisopropylamide (LDA) as in an aldol reaction. The thus formed enolate then gives a nucleophilic acyl substitution with the adjacent carbonyl of the acetyl group through a short lived intermediate oxirane. Acidic workup liberates the free hydroxyl group.
References
- ^ Rearrangement of α-acyloxyacetates into 2-hydroxy-3-ketoesters S. D. Lee, T. H. Chan, and K. S. Kwon Tetrahedron Lett. 1984, 25, 3399-3402. (doi:10.1016/S0040-4039(01)91030-5)
- ^ First total synthesis of taxol 1. Functionalization of the B ring Robert A. Holton, Carmen Somoza, Hyeong Baik Kim, Feng Liang, Ronald J. Biediger, P. Douglas Boatman, Mitsuru Shindo, Chase C. Smith, Soekchan Kim, et al.; J. Am. Chem. Soc. 1994, 116(4), 1597-1598. (doi:10.1021/ja00083a066)
See also
 |