Chemistry:Bamberger rearrangement
From HandWiki
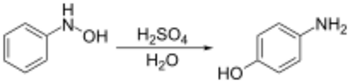
The Bamberger rearrangement is the chemical reaction of phenylhydroxylamines with strong aqueous acid, which will rearrange to give 4-aminophenols.[1] It is named for the German chemist Eugen Bamberger (1857–1932).[2][3]
The starting phenylhydroxylamines are typically synthesized by the transfer hydrogenation of nitrobenzenes using rhodium[4] or zinc[5] catalysts.
Application: Fenhexamide
Reaction mechanism
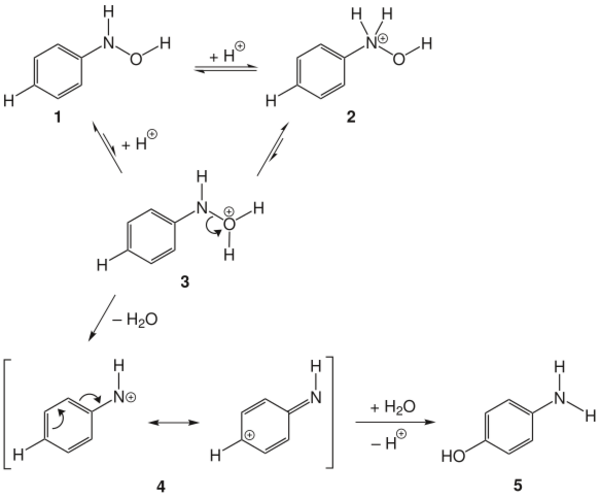
The mechanism of the Bamberger rearrangement proceeds from the monoprotonation of N-phenylhydroxylamine 1. N-protonation 2 is favored, but unproductive. O-protonation 3 can form the nitrenium ion 4, which can react with nucleophiles (H2O) to form the desired 4-aminophenol 5.[6][7]
See also
- Friedel–Crafts alkylation-like reactions:
- Hofmann–Martius rearrangement
- Fries rearrangement
- Fischer–Hepp rearrangement
- Wallach rearrangement
- Bamberger triazine synthesis — same inventor
References
- ↑ Harman, R. E. (1955). "Chloro-p-benzoquinone". Organic Syntheses 35: 22. http://www.orgsyn.org/Content/pdfs/procedures/CV4P0148.pdf.; Collective Volume, 4, pp. 148
- ↑ Bamberger, E. (1894). "Ueber die Reduction der Nitroverbindungen". Chemische Berichte 27 (2): 1347–1350. doi:10.1002/cber.18940270229. http://gallica.bnf.fr/ark:/12148/bpt6k907342/f163.
- ↑ Bamberger, E. (1894). "Ueber das Phenylhydroxylamin". Chemische Berichte 27 (2): 1548–1557. doi:10.1002/cber.18940270276. http://gallica.bnf.fr/ark:/12148/bpt6k907342/f376.table.
- ↑ Oxley, P. W.; Adger, B. M.; Sasse, M. J.; Forth1, M. A. (1989). "N-Acetyl-N-Phenylhydroxylamine Via Catalytic Transfer Hydrogenation of Nitrobenzene Using Hydrazine and Rhodium on Carbon". Organic Syntheses 67: 187. doi:10.15227/orgsyn.067.0187.
- ↑ Kamm, O. (1925). "β-Phenylhydroxylamine". Organic Syntheses 4: 57. http://www.orgsyn.org/orgsyn/prep.asp?prep=cv1p0445.; Collective Volume, 1, pp. 445 (download PDF)
- ↑ Sone, T.; Hamamoto, K.; Seiji, Y.; Shinkai, S.; Manabe, O. (1981). "Kinetics and Mechanisms of the Bamberger Rearrangement. Part 4. Rearrangement of Sterically Hindered Phenylhydroxylamines to 4-Aminophenols in Aqueous Sulphuric Acid Solution". Journal of the Chemical Society, Perkin Transactions 2 1981 (2): 1596–1598. doi:10.1039/P29810000298.
- ↑ Kohnstam, G.; Petch, W. A.; Williams, D. L. H. (1984). "Kinetic Substituent and Isotope Effects in the Acid-Catalysed Rearrangement of N-Phenylhydroxylamines. Are Nitrenium Ions Involved?". Journal of the Chemical Society, Perkin Transactions 2 1984 (3): 423–427. doi:10.1039/P29840000423.
 |