Chemistry:Prilezhaev reaction
| Prilezhaev reaction | |
|---|---|
| Named after | Nikolai Alexandrovich Prilezhaev (also spelled Nikolaj Alexandrovich Prileschajew, Russian: Николай Александрович Прилежаев) |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | prilezhaev-reaction |
| RSC ontology ID | RXNO:0000405 |
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides.[1] It is named after Nikolai Prilezhaev, who first reported this reaction in 1909.[2] A widely used peroxy acid for this reaction is meta-chloroperoxybenzoic acid (m-CPBA), due to its stability and good solubility in most organic solvents.[1][3] The reaction is performed in inert solvents (C
6H
14, C
6H
6, CH
2Cl
2, CHCl
3, CCl
4) between -10 and 60 °C with the yield of 60-80%.
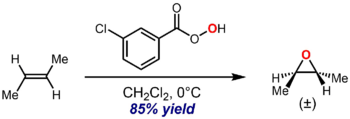
An illustrative example is the epoxidation of trans-2-butene with m-CPBA to give trans-2,3-epoxybutane:[4]
The oxygen atom that adds across the double bond of the alkene is taken from the peroxy acid, generating a molecule of the corresponding carboxylic acid as a byproduct. The reaction is highly stereospecific in the sense that the double bond stereochemistry is generally transferred to the relative configuration of the epoxide with essentially perfect fidelity, so that a trans-olefin leads to the stereoselective formation of the trans-2,3-substituted epoxide only, as illustrated by the example above, while a cis-olefin would only give the cis-epoxide. This stereochemical outcome is a consequence of the accepted mechanism, discussed below.
In general, the Prilezhaev reaction epoxidizes the most substituted double bond.[1]
Reaction mechanism
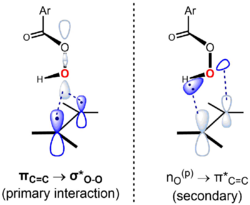
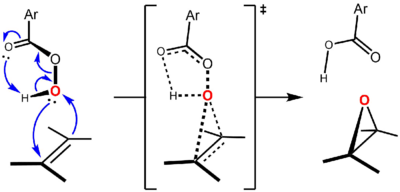
The reaction proceeds through what is commonly known as the "butterfly mechanism", first proposed by Bartlett, wherein the peracid is intramolecularly hydrogen-bonded at the transition state.[5] Although there are frontier orbital interactions in both directions, the peracid is generally viewed as the electrophile and the alkene as the nucleophile. In support of this notion, more electron-rich alkenes undergo epoxidation at a faster rate. For example, the relative rates of epoxidation increase upon methyl substitution of the alkene (the methyl groups increase the electron density of the double bond by hyperconjugation): ethylene (1, no methyl groups), propene (24, one methyl group), cis-2-butene (500, two methyl groups), 2-methyl-2-butene (6500, three methyl groups), 2,3-dimethyl-2-butene (>6500, four methyl groups).
The reaction is believed to be concerted, with a transition state that is synchronous or nearly so.[6] The "butterfly mechanism" takes place via a transition state geometry in which the plane of the peracid bisects that of the alkene, with the O–O bond aligned perpendicular to it. This conformation allows the key frontier orbital interactions to occur. The primary interaction of the occupied πC=C orbital (HOMO) and the low-lying unoccupied σ*O-O orbital (LUMO). This interaction accounts for the observed overall nucleophilic character and electrophilic character of the alkene and peracid, respectively. There is also a secondary interaction between a lone pair orbital perpendicular to the plane of the peracid, nO(p) (HOMO) and the unoccupied π*C=C orbital (LUMO).[7][8] Using the approach of Anslyn and Dougherty (2006, p. 556), the mechanism can be represented as follows:[9]
There is a very large dependence of the reaction rate on the choice of solvent.[10]
References
- ↑ 1.0 1.1 1.2 Li, Jie Jack; Corey, E. J. (2007). "Prilezhaev Reaction". Name Reactions of Functional Group Transformations. Wiley-Interscience. pp. 274–281. ISBN 9780470176504. https://books.google.com/books?id=WZ0DxnPNAdAC&pg=PA274.
- ↑ "Oxydation ungesättigter Verbindungen mittels organischer Superoxyde" (in German). Berichte der deutschen chemischen Gesellschaft 42 (4): 4811–4815. 1909. doi:10.1002/cber.190904204100. https://zenodo.org/record/1426367.
- ↑ Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press. p. 362. ISBN 978-0124297852.
- ↑ Vollhardt, K. Peter C.; Schore, Neil Eric (2011). Organic chemistry: Structure and function (6th ed.). New York: W.H. Freeman. ISBN 9781429204941. OCLC 422757611.
- ↑ Bartlett, Paul D. (1950). "Recent work on the mechanisms of peroxide reactions". Record of Chemical Progress 11: 47–51.
- ↑ Singleton, Daniel A.; Merrigan, Steven R.; Liu, Jian; Houk, Kendall N. (1997). "Experimental Geometry of the Epoxidation Transition State". Journal of the American Chemical Society 119 (14): 3385–3386. doi:10.1021/ja963656u.
- ↑ Most textbooks depict the reaction mechanism using only four curly arrows, so that the O–H bond is used as the source of electrons for the formation of the second C–O bond. Although such a depiction is formally correct, it is better to regard a pre-existing oxygen lone pair as the source of electrons for that bond (resulting in an additional arrow), in light of the discussion on frontier orbital interactions involved in the reaction. Anslyn and Dougherty (2006, p. 556) depicts the Prilezhaev reaction mechanism using the arrow pushing shown here and has a discussion on using proper electron sources in Appendix 5 ("Pushing Electrons," pp. 1061-1074).
- ↑ Evans, David A.; Myers, Andrew G. (2007). "Chemistry 206 & 215 – Advanced Organic Chemistry Lecture Notes: Lecture Notes, Problem Sets and Exams". Harvard Department of Chemistry and Chemical Biology. https://archive.org/details/EvansD.A.HarvardsAdvancedOrganicChemistry2003. Retrieved December 27, 2018.
- ↑ Anslyn, Eric V.; Dougherty, Dennis A. (2006). "Epoxidation". Modern Physical Organic Chemistry. University Science Books. pp. 555–556. ISBN 9781891389313. https://books.google.com/books?id=gY-Sxijk_tMC&pg=PA556.
- ↑ Dryuk, V. G. (1976). "The mechanism of epoxidation of olefins by peracids". Tetrahedron 32 (23): 2855–2866. doi:10.1016/0040-4020(76)80137-8.
 |