Medicine:Drug titration
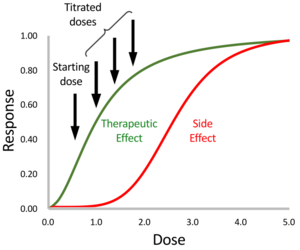
Drug titration is the process of adjusting the dose of a medication for the maximum benefit without adverse effects.[1]
When a drug has a narrow therapeutic index, titration is especially important, because the range between the dose at which a drug is effective and the dose at which side effects occur is small.[2] Some examples of the types of drugs commonly requiring titration include insulin, anticonvulsants, blood thinners, anti-depressants, and sedatives.[3][4][5]
Titrating off of a medication instead of stopping abruptly is recommended in some situations. Glucocorticoids should be tapered after extended use to avoid adrenal insufficiency.[6]
Drug titration is also used in phase I of clinical trials. The experimental drug is given in increasing dosages until side effects become intolerable.[7] A clinical trial in which a suitable dose is found is called a dose-ranging study.
See also
References
- ↑ "Chapter 2: Therapeutics and Good Prescribing: Choosing a Dosing Regime". Davidson's Principles and Practice of Medicine. Elsevier Health Sciences. 2013. pp. 34. ISBN 978-0-7020-5103-6. https://books.google.com/books?id=W-5kAgAAQBAJ&pg=PA34.
- ↑ "General Pharmacology". Clinical Pharmacology (11 ed.). Elsevier. 2012. pp. 74–109. ISBN 978-0-7020-4084-9.
- ↑ "Chapter 5 : Principles of Clinical Pharmacology". Principles of Clinical Pharmacology (19th ed.). New York, NY: McGraw-Hill. 2014. ISBN 978-0-07-180215-4.
- ↑ "Section III: Therapeutic Drugs and Antidotes". Poisoning & Drug Overdose (7th ed.). New York, NY: McGraw-Hill. 11 December 2017. ISBN 978-0-07-183979-2.
- ↑ "Chapter 27: Skeletal Muscle Relaxants". Basic & Clinical Pharmacology (14th ed.). New York, NY: McGraw-Hill. 30 November 2017. ISBN 978-1-259-64115-2.
- ↑ "Glucocorticoid withdrawal". Treatment Issues in Rheumatology. https://www.uptodate.com/contents/glucocorticoid-withdrawal. Retrieved 13 June 2018.
- ↑ "Dose-Response Information to Support Drug Registration". Guideline for Industry. FDA. November 1994. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073115.pdf. Retrieved 13 June 2018.
 |