Physics:Empirical valence bond
In theoretical chemistry, the Empirical Valence Bond (EVB) approach is an approximation for calculating free-energies of a chemical reaction in condensed-phase. It was first developed by Israeli chemist Arieh Warshel,[1] and was inspired by the way Marcus theory uses potential surfaces to calculate the probability of electron transfer.
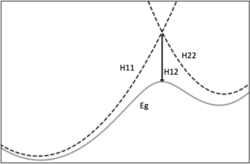
Where most methods for reaction free-energy calculations require at least some part of the modeled system to be treated using quantum mechanics, EVB uses a calibrated Hamiltonian to approximate the potential energy surface of a reaction. For a simple 1-step reaction, that typically means that a reaction is modeled using 2 states. These states are valence bond descriptions of the reactants and products of the reaction. The function that gives the ground energy then becomes:
[math]\displaystyle{ E_g = \frac{1}{2} \biggl(H_{11} + H_{22} - \sqrt{ (H_{11} - H_{22})^2 + 4H_{12}^2} \biggr) }[/math]
where H11 and H22 are the valence bond descriptions of the reactant and product state respectively, and H12 is the coupling parameter. The H11 and H22 potentials are usually modeled using force field descriptions Ureactants and Uproducts. H12 is a bit trickier as it needs to be parameterized using a reference reaction. This reference reaction can be experimental, typically from a reaction in water or other solvents. Alternatively quantum chemical calculations can be used for calibration.
Free energy calculations
To obtain free-energies from the created ground state energy potential one needs to perform sampling. This can be done using sampling methods like molecular dynamics or Monte Carlo simulations at different states along the reaction coordinates. Typically this is done using a free energy perturbation / umbrella sampling approach.[2]
Application
EVB has been successfully applied to calculating reaction free energies of enzymes.[2] More recently it has been looked at as a tool to study enzyme evolution[3] and to assist in enzyme design.[4]
Software
- Molaris
- Q
- RAPTOR (Rapid Approach for Proton Transport and Other Reactions)
See also
References
- ↑ Warshel, Arieh; Weiss, Robert M. (September 1980). "An empirical valence bond approach for comparing reactions in solutions and in enzymes" (in en). Journal of the American Chemical Society 102 (20): 6218–6226. doi:10.1021/ja00540a008. ISSN 0002-7863.
- ↑ 2.0 2.1 Warshel, Arieh. (1991). Computer modeling of chemical reactions in enzymes and solutions. New York: Wiley. ISBN 0-471-53395-5. OCLC 23016681.
- ↑ Åqvist, Johan; Isaksen, Geir Villy; Brandsdal, Bjørn Olav (July 2017). "Computation of enzyme cold adaptation" (in en). Nature Reviews Chemistry 1 (7): 0051. doi:10.1038/s41570-017-0051. ISSN 2397-3358. http://www.nature.com/articles/s41570-017-0051.
- ↑ Frushicheva, Maria P; Mills, Matthew JL; Schopf, Patrick; Singh, Manoj K; Prasad, Ram B; Warshel, Arieh (August 2014). "Computer aided enzyme design and catalytic concepts" (in en). Current Opinion in Chemical Biology 21: 56–62. doi:10.1016/j.cbpa.2014.03.022. PMID 24814389.
 |