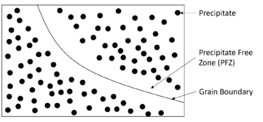
Physics:Precipitate-free zone
In materials science, a precipitate-free zone (PFZ) refers to microscopic localized regions around grain boundaries that are free of precipitates (solid impurities forced outwards from the grain during crystallization). It is a common phenomenon that arises in polycrystalline materials (crystalline materials with stochastically-oriented grains) where heterogeneous nucleation of precipitates is the dominant nucleation mechanism.[1][2][3] This is because grain boundaries are high-energy surfaces that act as sinks for vacancies, causing regions adjacent to a grain boundary to be devoid of vacancies.[4] As it is energetically favorable for heterogeneous nucleation to occur preferentially around defect-rich sites such as vacancies, nucleation of precipitates is impeded in the vacancy-free regions immediately adjacent to grain boundaries[4]
History
Pioneering studies on the theory[5] and experimental observation[6] of PFZs were made in the 1960s.
Effect on material properties
PFZs are detrimental to the mechanical properties of materials.[3] In particular, PFZs degrade the material's hardness, because the lack of precipitates in PFZs lead to these regions having fewer pinning sites. Dislocation motion – a condition necessary to cause a material to yield – will require an appreciably lower applied shear stress in PFZs, and consequently these locally weak zones will lead to plastic deformation.[7][8] The width of PFZs have also been found to be negatively correlated with intergranular fracture[1][7][8]
PFZs also accelerate pitting corrosion and stress corrosion cracking, significantly reducing the usable life of these materials in chemically aggressive environments.[9]
Techniques to minimize
It has been shown that PFZs can be minimized by quenching. First, quenching increases undercooling, favoring homogeneous nucleation in PFZs as it lowers the nucleation energy barrier even in the absence of potent nucleation sites. Additionally, low temperatures also lead to a reduction in diffusion rates, minimizing the loss of vacancies and premature growth of grain boundary precipitates.[5] However, since diffusion rates at low temperatures are suppressed, the aging time (time taken for treatment to yield a desired grain size) would be long. Therefore, one processing technique to circumvent this is to increase the temperature slightly once a sufficient number of homogeneous nucleation sites have been formed. Another technique to minimize PFZs is to introduce impurity elements, as they strongly interact with vacancies and allow for a more even distribution of vacancies in the material.[10][5][11] One example would be to introduce Mg in Al alloys[3]
Cyclic strengthening (CS), a process wherein a material is mechanically pushed and pulled repeatedly at room temperature, creates fine precipitates that is homogeneously distributed throughout the microstructure.[12] It has been suggested as an alternative to conventional, precipitate hardened alloys as this process achieves strengthening effects without introducing PFZs.
References
- ↑ 1.0 1.1 Sævareid, Sondre (2017). Influence of precipitate-free zones on tensile ductility and tear resistance of 6000 aluminium alloys - An experimental and numerical study. 108 (Master thesis).
- ↑ Maldonado, R.; Nembach, E. (1997-01-01). "The formation of precipitate free zones and the growth of grain boundary carbides in the nickel-base superalloy NIMONIC PE16" (in en). Acta Materialia 45 (1): 213–224. doi:10.1016/S1359-6454(96)00139-5. ISSN 1359-6454. Bibcode: 1997AcMat..45..213M. https://www.sciencedirect.com/science/article/pii/S1359645496001395.
- ↑ 3.0 3.1 3.2 Song, Jie; Field, Robert; Konitzer, Doug; Kaufman, Michael (2017-05-01). "Development of Grain Boundary Precipitate-Free Zones in a Ni-Mo-Cr-W Alloy" (in en). Metallurgical and Materials Transactions A 48 (5): 2425–2434. doi:10.1007/s11661-017-4019-8. ISSN 1543-1940. Bibcode: 2017MMTA...48.2425S. https://doi.org/10.1007/s11661-017-4019-8.
- ↑ 4.0 4.1 Chen, Y. Q.; Pan, S. P.; Tang, S. W.; Liu, W. H.; Tang, C. P.; Xu, F. Y. (2016-08-01). "Formation mechanisms and evolution of precipitate-free zones at grain boundaries in an Al–Cu–Mg–Mn alloy during homogenisation" (in en). Journal of Materials Science 51 (16): 7780–7792. doi:10.1007/s10853-016-0062-x. ISSN 1573-4803. Bibcode: 2016JMatS..51.7780C. https://doi.org/10.1007/s10853-016-0062-x.
- ↑ 5.0 5.1 5.2 Embury, J. D; Nicholson, R. B (1965-04-01). "The nucleation of precipitates: The system Al-Zn-Mg" (in en). Acta Metallurgica 13 (4): 403–417. doi:10.1016/0001-6160(65)90067-2. ISSN 0001-6160. https://dx.doi.org/10.1016/0001-6160%2865%2990067-2.
- ↑ Unwin, P. N. T; Lorimer, G. W; Nicholson, R. B (1969-11-01). "The origin of the grain boundary precipitate free zone" (in en). Acta Metallurgica 17 (11): 1363–1377. doi:10.1016/0001-6160(69)90154-0. ISSN 0001-6160. https://dx.doi.org/10.1016/0001-6160%2869%2990154-0.
- ↑ 7.0 7.1 Krol, Thorsten; Baither, Dietmar; Nembach, Eckhard (2003-04-14). "Quantification of the detrimental effects of precipitate free zones on the yield strength of a superalloy" (in en). Scripta Materialia. ViewPoint Set No. 29 "Phase Transformations and Deformations in Magnesium Alloys" 48 (8): 1189–1194. doi:10.1016/S1359-6462(02)00566-3. ISSN 1359-6462. https://www.sciencedirect.com/science/article/pii/S1359646202005663.
- ↑ 8.0 8.1 Ogura, Tomo; Hirosawa, Shoichi; Cerezo, Alfred; Sato, Tatsuo (2010-10-01). "Atom probe tomography of nanoscale microstructures within precipitate free zones in Al–Zn–Mg(–Ag) alloys" (in en). Acta Materialia 58 (17): 5714–5723. doi:10.1016/j.actamat.2010.06.046. ISSN 1359-6454. Bibcode: 2010AcMat..58.5714O. https://www.sciencedirect.com/science/article/pii/S1359645410004118.
- ↑ Talbot, David E. J.; Talbot, James D. R. (2018-01-29) (in en). Corrosion Science and Technology. CRC Press. ISBN 978-1-4987-5242-8. https://books.google.com/books?id=PmpQDwAAQBAJ&dq=D.+E.+J.+Talbot%2C+J.+D.+R.+Talbot%2C+Corrosion+Science+and+Technology+%28CRC+Press%2C+ed.+3%2C+2018%29.&pg=PP1.
- ↑ Yao, D. P.; Zhang, Y. Z.; Hu, Z. Q.; Li, Y. Y.; Shi, C. X. (1989-04-01). "The formation and growth of PFZ at grain boundary in Al-11.9at.-%Li alloy" (in en). Scripta Metallurgica 23 (4): 537–541. doi:10.1016/0036-9748(89)90447-X. ISSN 0036-9748. https://dx.doi.org/10.1016/0036-9748%2889%2990447-X.
- ↑ Tzeng, Yu-Chih; Wu, Chih-Ting; Lee, Sheng-Long (2015-12-15). "The effect of trace Sc on the quench sensitivity of AL–7Si–0.6 Mg alloys" (in en). Materials Letters 161: 340–342. doi:10.1016/j.matlet.2015.08.108. ISSN 0167-577X. https://www.sciencedirect.com/science/article/pii/S0167577X1530464X.
- ↑ Sun, Wenwen; Zhu, Yuman; Marceau, Ross; Wang, Lingyu; Zhang, Qi; Gao, Xiang; Hutchinson, Christopher (March 2019). "Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity" (in en). Science 363 (6430): 972–975. doi:10.1126/science.aav7086. ISSN 0036-8075. PMID 30819960. Bibcode: 2019Sci...363..972S.
 |