Chemistry:Claisen-Schmidt condensation
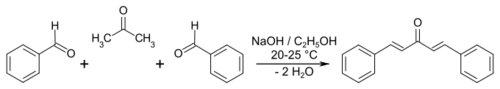
In organic chemistry, the Claisen–Schmidt condensation is the reaction between an aldehyde or ketone having an α-hydrogen with an aromatic carbonyl compound lacking an α-hydrogen. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. G. Schmidt, who independently published on this topic in 1880 and 1881.[1][2][3][page needed] An example is the synthesis of dibenzylideneacetone ((1E, 4E)-1,5-diphenylpenta-1,4-dien-3-one).[4]
Quantitative yields in Claisen–Schmidt reactions have been reported in the absence of solvent using sodium hydroxide as the base and plus benzaldehydes.[5] Because the enolizeable nucleophilic carbonyl compound and the electrophilic carbonyl compound are two different chemicals, the Claisen–Schmidt reaction is an example of a crossed aldol process.
References
- ↑ Claisen, L.; Claparède, A. (1881). "Condensationen von Ketonen mit Aldehyden". Berichte der Deutschen Chemischen Gesellschaft 14 (1): 2460–2468. doi:10.1002/cber.188101402192. http://gallica.bnf.fr/ark:/12148/bpt6k906939/f871.chemindefer.
- ↑ Schmidt, J. G. (1881). "Ueber die Einwirkung von Aceton auf Furfurol und auf Bittermandelöl in Gegenwart von Alkalilauge". Berichte der Deutschen Chemischen Gesellschaft 14 (1): 1459–1461. doi:10.1002/cber.188101401306. http://gallica.bnf.fr/ark:/12148/bpt6k90692z/f1461.chemindefer.
- ↑ March, J. (1985). Advanced Organic Chemistry: Reactions, Mechanisms and Structure (3rd ed.). Wiley Interscience. ISBN 0-471-85472-7.
- ↑ Hull, L. A. (February 2001). "The Dibenzalacetone Reaction Revisited". J. Chem. Educ. 78 (2): 226. doi:10.1021/ed078p226. Bibcode: 2001JChEd..78..226H. https://pubs.acs.org/doi/abs/10.1021/ed078p226.
- ↑ Rahman A. F. M. Motiur, Ali Roushown, Jahng Yurngdong, Kadi Adnan A. (2012). "A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones". Molecules 17 (1): 571–583. doi:10.3390/molecules17010571. PMID 22231494.