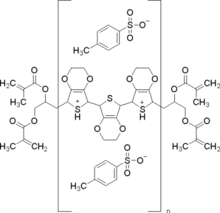
Chemistry:PEDOT-TMA
| |
| Names | |
|---|---|
| Other names
Oligotron; Pedot tetramethacrylate; Poly(3,4-ethylenedioxythiophene), tetramethacrylate end-capped, PEDOT-TM, Meth-Pedot, Pedot-Meth
| |
| Identifiers | |
| Properties | |
| Molar mass | ~6000 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Poly(3,4-ethylenedioxythiophene)-tetramethacrylate or PEDOT-TMA is a p-type conducting polymer based on 3,4-ethylenedioxylthiophene or the EDOT monomer. It is a modification of the PEDOT structure. Advantages of this polymer relative to PEDOT (or PEDOT) are that it is dispersible in organic solvents, and it is non-corrosive. PEDOT-TMA was developed under a contract with the National Science Foundation, and it was first announced publicly on April 12, 2004.[1] The trade name for PEDOT-TMA is Oligotron. PEDOT-TMA was featured in an article entitled "Next Stretch for Plastic Electronics" that appeared in Scientific American in 2004.[2][3] The U.S. Patent office issued a patent protecting PEDOT-TMA on April 22, 2008.[4]
PEDOT-TMA differs from the parent polymer PEDOT in that it is capped on both ends of the polymer. This limits the chain-length of the polymer, making it more soluble in organic solvents than PEDOT. The methacrylate groups on the two end-caps allow further chemistry to occur such as cross-linking to other polymers or materials.
Physical properties
The bulk conductivity of PEDOT-TMA is 0.1-.5 S/cm, the sheet resistance 1-10 M Ω/sq, and the methacrylate equivalent weight 1360-1600 g/mol. The chemical composition of a film of PEDOT-TMA was measured by energy-dispersive x-ray spectroscopy (EDS). The relative C, O, and S weight percentages were 51.28%, 35.37%, and 10.43%. There was also 2.92% Fe present in the film.[5]
Applications
Several devices and materials have been described in both journals and the patent literature that use PEDOT-TMA as a critical component. In this section, a brief overview of these inventions is given.
- Patternable OLEDs: In a study[6] by researchers at General Electric, PEDOT-TMA was used in the hole injection layer in a series of OLED devices. They have also filed a patent application to protect this invention.[7]
- Quantum dot modified OLEDs: In an international patent application, PEDOT-TMA surfaces were modified with quantum dots such as CdSe, CdS, and ZnS.[8]
- Ion selective membranes: PEDOT-TMA was used as a key ingredient in ion selective membranes[9] and in particular in calcium-selective electrodes.[10] The performance of PEDOT-TMA films in solid contact ion selective electrodes compared to other commercially available conducting polymers has also been reported.[11]
- Dye sensitized solar cell: PEDOT-TMA was used in the construction of effective Dye-sensitized solar cells.[12][13] The PEDOT-TMA was spun-coat to give a 15 nm thick layer which was used as the counter-electrode in a series of Dye-sensitized solar cells. Efficiencies as high as 7.85% were obtained.[14][15][16]
- Flexible touch screens: PEDOT-TMA was used in the construction of electrodes for flexible touch screens as described in a patent application by the Honeywell Corporation.[17]
- Energy storage and conversion devices: Synkera Technologies, Inc. filed a patent application detailing a variety of energy storage and conversion devices that use PEDOT-TMA in their construction.[18]
- Glucose sensor: A glucose sensor was prepared by Gymama Slaughter of Virginia State University.[19]
- Carbon nanotube composites: Researchers from Los Alamos National Laboratory used PEDOT-TMA to prepare composites with carbon nanotubes. These composites form highly aligned arrays of the nanotubes, and exhibit high conductivity at room temperature (25.0 S/cm).[20]
- Metal wire-based photovoltaic device: Researchers from The Institute of Advanced Energy at Kyoto University used PEDOT-TMA to fabricate organic photovoltaic devices.[21]
- Embedded capacitors: Researchers from The Polymer Composite Laboratory at VIT University prepared composites of Graphene oxide with PEDOT-TMA and PMMA. They extensively studied the properties of these materials as a function of Graphene oxide composition. The materials were characterized by UV-Vis spectroscopy, FT-IR and FT-Raman spectroscopy, X-Ray diffraction, thermogravimetric analysis, atomic force microscopy and scanning electron microscopy. Finally, the dielectric properties of the materials were evaluated, and the potential application of the composites in constructing embedded capacitors was discussed.[22] This research group has also developed thermistors made from Graphene Oxide/PEDOT-TMA composites.[23]
- Titanium dioxide nanocomposites: A research group led by A.A.M. Farag has prepared and characterized nanocomposites of TiO2[24] and ZnO[25] with PEDOT-TMA. This group has also prepared and characterized heterojunction diodes using this nanocomposite.[26]
- Ultrathin Fiber-Mesh Polymer Thermistors: Ultrathin fibers were prepared that show a 10^3 increase in resistance over a narrow temperature range suitable for on-skin and implantable sensors. These thermistors prevent overheating in devices that use thermal protection circuits.[27]
References
- ↑ Chamot, J. (April 12, 2004). "New Molecule Heralds Breakthrough in Electronic Plastics". https://www.nsf.gov/news/news_summ.jsp?cntn_id=100360.
- ↑ Collins, Graham P. (August 1, 2004). "Next Stretch for Plastic Electronics". Scientific American 291 (2): 75–81. doi:10.1038/scientificamerican0804-74. PMID 15298122. Bibcode: 2004SciAm.291b..74C.
- ↑ "Light and Magic". The Economist: p. 74. 2004-05-22. http://www.economist.com/node/2685725.
- ↑ Elliott; Brian J.; Luebben; Silvia D. & Sapp; Shawn A. et al., "Electrically conducting materials from branched end-capping intermediates", US patent 7361728, published 2008-04-22, assigned to TDA Research, Inc.
- ↑ He, Jiarong; Jing Su; Jinglun Wang; Lingzhi Zhang (2018). "Synthesis of water-free PEDOT with polyvinylpyrrolidone stabilizer in organic dispersant system". Organic Electronics 53: 117–126. doi:10.1016/j.orgel.2017.11.035.
- ↑ Liu, J.; L. N. Lewis; A. R. Dugal (2007). "Photoactivated and patternable charge transport materials and their use in organic light-emitting devices". Appl. Phys. Lett. 90 (23): 233503. doi:10.1063/1.2746404. Bibcode: 2007ApPhL..90w3503L.
- ↑ Liu, Jie; Larry Neil Lewis; Anil Raj Duggal; Rubinsztajn Slawomir (2005-10-04). US Patent Application US 2007/0077452, Organic light emitting devices having latent activated layers and methods of fabricating the same.
- ↑ Vitukhnovskii, Alexey; Andrey Vashenko; Denis Bychkovskii (2014-12-31). WO Patent Application 2014/209154A1, Organic light-emitting element with the radiating layer containing quantum dots with modified surface.
- ↑ Rzewuska, Anna; Marcin Wojciechowski; Ewa Bulska; Elizabeth A. H. Hall; Krzysztof Maksymiuk; Agata Michalska (2008). "Composite Polyacrylate-Poly(3,4- ethylenedioxythiophene) Membranes for Improved All-Solid-State Ion-Selective Sensors". Anal. Chem. 80 (1): 321–327. doi:10.1021/ac070866o. PMID 18062675.
- ↑ Ocana Tejada, Cristina; Natalia Abramova; Andrey Bratov; Tom Lindfors; Johan Bobacka (2018). "Calcium-selective electrodes based on photo-cured polyurethane-acrylate membranes covalently attached to methacrylate functionalized poly(3,4-ethylenedioxythiophene) as a solid-contact". Talanta 186: 279–285. doi:10.1016/j.talanta.2018.04.056. PMID 29784361. http://urn.fi/URN:NBN:fi-fe2020100883135.
- ↑ Ocana, C.; M. Munoz-Correas; N. Abramova; A. Bratov (2020). "Comparison of Different Commercial Conducting Materials as Ion-to-Electron Transducer Layers in Low-Cost Selective Solid-Contact Electrodes". Sensors 20 (5): 1348–1360. doi:10.3390/s20051348. PMID 32121463. Bibcode: 2020Senso..20.1348O.
- ↑ Kim, Kyung Ho; Takashi Okubo; Naoyo Tanaka; Naoto Mimura; Masahiko Maekawa; Takayoshi Kuroda-Sowa (2010). "Dye-sensitized Solar Cells with Halide-bridged Mixed-valence Cu(I)-Cu(II) Coordination Polymers with Hexamethylenedithiocarbamate Ligand". Chem. Lett. 39 (7): 792–793. doi:10.1246/cl.2010.792.
- ↑ Okubo, Takashi; Naoyo Tanaka; Haruho Anma Kyung; Ho Kim; Masahiko Maekawa; Takayoshi Kuroda-Sowa (2012). "Dye-sensitized Solar Cells with New One-Dimensional Halide-Bridged Cu(I)–Ni(II) Heterometal Coordination Polymers Containing Hexamethylene Dithiocarbamate Ligand". Polymers 4 (3): 1613–1626. doi:10.3390/polym4031613.
- ↑ Kim, Kyung Ho; Kazuomi Utashiro; Zhuguang Jin; Yoshio Abe; Midori Kawamura (2013). "Dye-Sensitized Solar Cells with Sol-Gel Solution Processed Ga-Doped ZnO Passivation Layer". Int. J. Electrochem. Sci. 8 (4): 5183–5190. doi:10.1016/S1452-3981(23)14672-4.
- ↑ Kim, Kyung Ho; Kazuomi Utashiro; Yoshio Abe; Midori Kawamura (2014). "Structural Properties of Zinc Oxide Nanorods Grown on Al-Doped Zinc Oxide Seed Layer and Their Applications in Dye-Sensitized Solar Cells". Materials 7 (4): 2522–2533. doi:10.3390/ma7042522. PMID 28788581. Bibcode: 2014Mate....7.2522K.
- ↑ Yoshimura, Nobutaka; Atsushi Kobayashi; Wataru Genno; Takashi Okubo; Masaki Yoshida; Masako Kato (2020). "Photosensitizing Ruthenium(II)-Dye Multilayers: Photoinduced Charge Separation and Back Electron Transfer Suppression". Sustainable Energy & Fuels 4 (7): 3450–3457. doi:10.1039/D0SE00151A.
- ↑ Edwards, Lewin; Patricia McCrimmon; Richard Thomas Watson (2010-07-22). US Patent Application 2010/0182245, Tactile-Feedback Touch Screen.
- ↑ Routkevitch, Dmitri; Rikard A. Wind (2010-12-02). US Patent Application 2010/0304204, Energy Conversion and Energy Storage Devices and Methods for Making Same.
- ↑ Slaughter, Gymama (2010). "Fabrication of Nanoindented Electrodes for Glucose Detection". J. Diabetes Sci. Technol. 4 (2): 320–327. doi:10.1177/193229681000400212. PMID 20307392.
- ↑ Peng, Huisheng; Xuemei Sun (2009). "Highly Aligned Carbon Nanotube/Polymer Composites with Much Improved Electrical Conductivities". Chemical Physics Letters 471 (1–3): 103–105. doi:10.1016/j.cplett.2009.02.008. Bibcode: 2009CPL...471..103P.
- ↑ Chuangchote, Surawut; Takashi Sagawaa; Susumu Yoshikawa (2011). "Design of metal wires-based organic photovoltaic cells". Energy Procedia 9: 553–558. doi:10.1016/j.egypro.2011.09.064. https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/235662/1/j.egypro.2011.09.064.pdf.
- ↑ Deshmukh, Kalim; Girish M. Joshi (2015). "Embedded capacitor applications of grapheme oxide reinforced poly(3,4-ethylenedioxythiophene)-tetramethacrylate (PEDOT-TMA) composites". Journal of Materials Science: Materials in Electronics 26 (8): 5896–5909. doi:10.1007/s10854-015-3159-0.
- ↑ Joshi, Girish; Kalim Deshmukh (2015). "Conjugated Polymer/Graphene oxide Nanocomposite As Thermistor". AIP Conference Proceedings 1665 (1): 050017. doi:10.1063/1.4917658. Bibcode: 2015AIPC.1665e0017J.
- ↑ Ashery, A.; G. Said; W.A. Arafa; A.E.H. Gaballah; A.A.M. Farag (2016). "Morphological and crystalline structural characteristics of PEDOT/TiO2 nanocomposites for applications towards technology in electronic devices". Journal of Alloys and Compounds 671: 291–298. doi:10.1016/j.jallcom.2016.02.088.
- ↑ Ashery, A.; A.A.M. Farag; A.E.H. Gaballah; G. Said; W.A. Arafa (2017). "Nanostructural, optical and heterojunction characteristics of PEDOT/ZnO nanocomposite thin films". Journal of Alloys and Compounds 723: 276–287. doi:10.1016/j.jallcom.2017.06.260.
- ↑ Ashery, A.; G. Said; W.A. Arafa; A.E.H. Gaballah; A.A.M. Farag (2016). "Structural and optical characteristics of PEDOT/n-Si heterojunction diode". Synthetic Metals 214: 92–99. doi:10.1016/j.synthmet.2016.01.008.
- ↑ Okutani, Chihiro; Tomoyuki Yokota; Takeo Someya (2022). "Ultrathin Fiber-Mesh Polymer Thermistors". Advanced Science 9 (30): e2202312. doi:10.1002/advs.202202312. PMID 36057993.
 |

