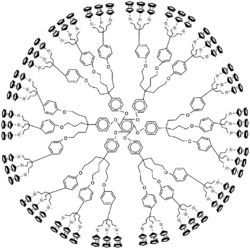
Chemistry:Metallodendrimer
A metallodendrimer is a type of dendrimer with incorporated metal atoms. The development of this type of material is actively pursued in academia.[1][2][3]
Structure
The metal can be situated in the repeat unit, the core or at the extremities as end-group. Elements often encountered are palladium and platinum. These metals can form octahedral six-coordinate M(IV) linking units from organic dihalides and the corresponding 4-coordinate M(II) monomers. Ferrocene-containing dendrimers and dendrimers with cobaltocene and arylchromiumtricarbonyl units have been reported in end-functional dendrimers.
Metallodendrimers can form as metal complexes with dendritic counter ions for example by hydrolysis of ester terminated PAMAM dendrimers with sodium hydroxide.
Applications
Metallodendrimers are investigated as equivalents to nanoparticles. Applications can be expected in the fields of catalysis, as chemical sensors in molecular recognition - for example of bromine and chloride anions [4] - or as materials capable of binding metals. Metallodendrimers can also mimic certain biomolecules for example haemoprotein in dendrimer with a porphyrin core. Further uses are reported as electrocatalyst.[5][6]
Examples of metallodendrimer heterogeneous catalysis are a nickel-containing dendrimer active in the Kharasch addition,[7] palladium-containing dendrimers active in ethylene polymerization[8] and in the Heck reaction.[9]
References
- ↑ Gorman, C. (1998). "Metallodendrimers: Structural Diversity and Functional Behavior". Advanced Materials 10 (4): 295–309. doi:10.1002/(SICI)1521-4095(199803)10:4<295::AID-ADMA295>3.0.CO;2-N.
- ↑ Cuadrado, I.; Morán, M. S.; Casado, C. M.; Alonso, B.; Losada, J. (1999). "Organometallic dendrimers with transition metals". Coordination Chemistry Reviews 193-195: 395–445. doi:10.1016/S0010-8545(99)00036-3.
- ↑ Stoddart, F.; Welton, T. (1999). "Metal-containing dendritic polymers". Polyhedron 18 (27): 3575. doi:10.1016/S0277-5387(99)00301-0.
- ↑ Valério, C.; Alonso, E.; Ruiz, J.; Blais, J. C.; Astruc, D. (1999). "A Polycationic Metallodendrimer with 24 [Fe(η5-C5Me5)(η6-N-Alkylaniline)]+ Termini That Recognizes Chloride and Bromide Anions". Angewandte Chemie International Edition 38 (12): 1747. doi:10.1002/(SICI)1521-3773(19990614)38:12<1747::AID-ANIE1747>3.0.CO;2-G.
- ↑ Cheng, L.; Cox, J. A. (2002). "Nanocomposite Multilayer Film of a Ruthenium Metallodendrimer and a Dawson-Type Polyoxometalate as a Bifunctional Electrocatalyst". Chemistry of Materials 14: 6–8. doi:10.1021/cm010854y.
- ↑ Cheng, L.; Pacey, G. E.; Cox, J. A. (2001). "Carbon Electrodes Modified with Ruthenium Metallodendrimer Multilayers for the Mediated Oxidation of Methionine and Insulin at Physiological pH". Analytical Chemistry 73 (22): 5607–5610. doi:10.1021/ac0105585. PMID 11816594.
- ↑ Knapen, J. W. J.; Van Der Made, A. W.; De Wilde, J. C.; Van Leeuwen, P. W. N. M.; Wijkens, P.; Grove, D. M.; Van Koten, G. (1994). "Homogeneous catalysts based on silane dendrimers functionalized with arylnickel(II) complexes". Nature 372 (6507): 659. doi:10.1038/372659a0. Bibcode: 1994Natur.372..659K.
- ↑ Smith, G.; Chen, R.; Mapolie, S. (2003). "The synthesis and catalytic activity of a first-generation poly(propylene imine) pyridylimine palladium metallodendrimer". Journal of Organometallic Chemistry 673 (1–2): 111–035. doi:10.1016/S0022-328X(03)00173-6.
- ↑ Smith, G.; Mapolie, S. F. (2004). "Iminopyridyl-palladium dendritic catalyst precursors: evaluation in Heck reactions". Journal of Molecular Catalysis A: Chemical 213 (2): 187–192. doi:10.1016/j.molcata.2003.12.010.
 |