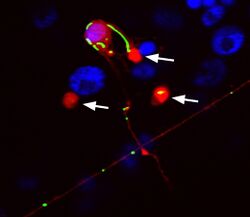
Biology:Exopher
Exophers are a type of membrane-bound extracellular vesicle (EV) that are released by budding out of cells into the extracellular space. Exophers can be released by neurons[1] and muscle[2] in the nematode Caenorhabditis elegans and also from murine cardiomyocytes.[3] Exophers are notable for their large size, averaging approximately four microns in diameter, and they are able to expel whole organelles, such as mitochondria and lysosomes as cargo.[1] An exopher can initially remain attached to the cell that produced it by a membranous filament that resembles a tunneling nanotube. Exophers share similarities with large oncosomes, but they differ in that they are produced by physiologically normal cells instead of aberrant cells associated with tumors.[4]
Exopher production is thought to be a mechanism cells use to preserve homeostasis. Exophers are produced in response to numerous stressors including intracellular protein aggregation, reactive oxygen species (ROS),[1] heat, osmotic hyertonicity, starvation,[5] and even space flight.[6] Mechanistically, exopher production has been found to depend on extracellular receptor signaling. Two MAPK pathways, epidermal growth factor (EGF) and fibroblast growth factor (FGF) signaling have been implicated in exopher production in nematodes.[5] Extracellular signaling receptor MERTK, expressed by cardiac-resident macrophages, is necessary for exopher clearance by phagocytosis in mouse-derived cardiac tissue.[3]
Exophers may be relevant to disease. In mouse heart, eliminating macrophages or blocking their ability to engulf exophers lead to inflammation and ventricular dysregulation.[3] Exophers may also promote pathological protein spreading in neurodegenerative diseases due to their ability to carry aggregated proteins outside of neurons, including human huntingtin protein.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Melentijevic, I; Toth, ML; Arnold, ML; Guasp, RJ; Harinath, G; Nguyen, KC; Taub, D; Parker, JA et al. (2017). "C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress". Nature 542(7641) (7641): 367–371. doi:10.1038/nature21362. PMID 28178240. Bibcode: 2017Natur.542..367M.
- ↑ Turek, M; Banasiak, K; Piechota, M; Shanmugam, N; Macias, M; Śliwińska, MA; Niklewicz, M; Kowalski, K et al. (2021). "Muscle-derived exophers promote reproductive fitness". EMBO Rep. 22 (8): e52071. doi:10.15252/embr.202052071. PMID 34288362.
- ↑ 3.0 3.1 3.2 "A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart". Cell 183 (1): 94–109. 2020. doi:10.1016/j.cell.2020.08.031. PMID 32937105.
- ↑ "Oncosomes - large and small: what are they, where they came from?". Journal of Extracellular Vesicles 5: 33109. 2016. doi:10.3402/jev.v5.33109. PMID 27680302.
- ↑ 5.0 5.1 Cooper, JF; Guasp, RJ; Arnold, ML; Grant, BD; Driscoll, M (2021). "Stress increases in exopher-mediated neuronal extrusion require lipid biosynthesis, FGF, and EGF RAS/MAPK signaling". Proc Natl Acad Sci USA 118 (36): e2101410118. doi:10.1073/pnas.2101410118. PMID 34475208.
- ↑ "Spaceflight affects neuronal morphology and alters transcellular degradation of neuronal debris in adult Caenorhabditis elegans". iScience 24 (2): 102105. 2021. doi:10.1016/j.isci.2021.102105. PMID 33659873. Bibcode: 2021iSci...24j2105L.
 |