Company:Masimo
 | |
| Type | Public |
|---|---|
| NASDAQ: MASI S&P 400 Component | |
| Industry | Medical Technology Medical Devices Medical Equipment |
| Founder | Joe E. Kiani |
| Headquarters | Irvine, California 92618 , United States of America 92618 |
Area served | Worldwide |
| Revenue | |
Number of employees | 3,500 (2014) |
| Website | www |
Masimo is an American manufacturer of noninvasive patient monitoring technologies based in Irvine, California.[2] The company sells more pulse oximetry to hospitals than any other company.[3] Masimo was founded in 1989 by electrical engineer Joe Kiani, who was later joined by fellow engineer Mohamed Diab.[4] Masimo invented measure-through motion and low perfusion pulse oximetry, known as Masimo SET (Signal Extraction Technology). Masimo has been recognized for its intellectual property and for being one of the most innovative companies in the medical device industry.[5] The company went public in 2007[6] and is currently traded on the NASDAQ stock exchange under the symbol MASI. In 2011, Forbes named Masimo to its list of top 20 public companies under a billion dollars in revenue, based on earnings growth, sales growth, and return on equity.[7][8] In 2012, Joe Kiani, founder, CEO and Chairman of the Board was named the Ernst & Young National Entrepreneur of the Year - 2012 Life Sciences Award Winner. Kiani was recognized for "revolutionizing the health care industry by taking risks to create and commercialize noninvasive patient monitoring devices, which include an array of sensors that lead to improved accuracy, a reduction in the overall number of false readings, and ultimately, reduced cost of care."[9]
Technology
Signal Extraction Technology (SET) pulse oximetry
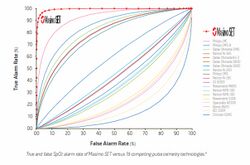
Pulse oximetry uses two light emitting diodes (LEDs), one red and one infrared, to measure the absorption of light and translates that into the percentage of hemoglobin molecules that are bound with oxygen, which is called arterial oxygen saturation (SpO2). Conventional pulse oximetry assumes that arterial blood is the only blood moving (pulsating) in the measurement site. However, during patient motion, the venous blood also moves, which can cause conventional pulse oximetry to under-read SpO2 levels because it cannot distinguish between the arterial and venous blood.[11][12] SET identifies the venous blood signal (which has a lower oxygen saturation level than arterial blood), isolates it, and uses adaptive filters to cancel the noise and extract the arterial signal in order to report accurate SpO2 and pulse rate. In addition, SET pulse oximetry provides perfusion index (PI) and pleth variability index (PVI). Multiple studies have shown that compared to non-SET pulse oximeters, SET increases the ability to detect life-threatening events and reduces false alarms during challenging conditions.[13][10] Additional studies have also shown the impact of SET on patient outcomes, such as helping clinicians:
- Decrease retinopathy of prematurity (ROP) in neonates[14][15]
- Increase detection of critical congenital heart disease (CCHD) in newborns[16]
- Reduce ventilator weaning time by titrating FiO2 faster and reduce arterial blood gas measurements in the Intensive Care Unit (ICU)[17]
- Decrease rapid response activations and Intensive Care Unit (ICU) transfers to save lives and costs in post-surgical patients on the medical-surgical floors through earlier identification of patients in distress through low SpO2 and abnormal pulse rate measurements[18][19]
- Decrease fluid administered during surgery and reduce patient risk[20]
In 2011, the American Academy of Pediatrics and the U.S. Department of Health and Human Services recommended mandatory screening for all newborns, using "motion-tolerant pulse oximeters that report functional oxygen saturation have been validated in low perfusion conditions".[21] To make this recommendation, the CCHD workgroup relied on two independent studies that exclusively used SET pulse oximetry to assess newborns. In 2012, Masimo received FDA 510(k) clearance for devices and sensors with labeling for screening newborns for CCHD. It marked the first time the FDA cleared specific labeling indicating the use of pulse oximeters, in conjunction with a physical examination, to screen newborns for CCHD.[22] In 2012, the National Health Service (NHS) Technology Adoption Centre in the United Kingdom advised hospitals to use Intraoperative Fluid Management Technologies as a way to improve patient outcomes, and included Masimo's PVI among technologies available for helping clinicians manage fluid during surgeries.[23] In 2013, the French Society for Anaesthesia and Intensive Care (SFAR) added PVI to its guidelines for optimal hemodynamic management of surgical patients.[24]
rainbow Pulse CO-Oximetry
rainbow Pulse CO-Oximetry uses more than seven wavelengths of light to continuously and noninvasively measure hemoglobin (SpHb), carboxyhemoglobin (SpCO), and methemoglobin (SpMet), in addition to oxygen saturation (SpO2), pulse rate, perfusion index (Pi), and pleth variability index (PVi).[25] A study at Massachusetts General Hospital showed that SpHb monitoring helped clinicians decrease the frequency of patients receiving blood transfusions during surgery from 4.5% to 0.6%.[26] Another study from Cairo University showed that SpHb monitoring helped clinicians reduce blood transfusions in high blood loss surgery by an average of 0.9 units per patient.[27] Emergency department studies have shown that SpCO helps clinicians increase the detection of carbon monoxide (CO) poisoning and decreases the time to treatment compared to invasive methods.[28][29][30] The Pronto-7 device for noninvasive spot checking of hemoglobin, along with SpO2 and pulse rate, has been recognized with a gold Medical Design Excellence Award.[31] The World Health Organization called noninvasive hemoglobin an "innovative medical technology for cost-effectively addressing global health concerns and needs".[32]
In October 2014, Masimo announced CE Mark of Oxygen Reserve Index or ORi, the company’s 11th noninvasive parameter, which provides real-time visibility to oxygenation status. ORi is intended to supplement, not replace, oxygen saturation (SpO2) monitoring and partial pressure of oxygen (PaO2) measurements. ORi can be trended and has optional alarms to notify clinicians of changes in a patient’s oxygen reserve, and may enable proactive interventions to avoid hypoxia and unintended hyperoxia.
Noninvasive Patient Monitoring
Patient SafetyNet
Patient SafetyNet is a remote monitoring and notification system designed for patients on medical/surgical care floors.[33] A large study by Dartmouth-Hitchcock Medical Center showed Patient SafetyNet helped clinicians achieve a 65% reduction in distress codes and rescue activations and a 48% decrease in patient transfers to intensive care units (ICU), yielding a savings of 135 Intensive Care Unit (ICU) days annually for an annual opportunity-cost savings of $1.48 million.[34][35] ECRI Institute gave Dartmouth its Health Devices Achievement Award for its use of Patient SafetyNet to prevent "severe patient harm".[36] Masimo has introduced Halo Index in the Patient SafetyNet system, combining multiple physiologic parameters into one number to help clinicians assess overall patient status.[37]
Rainbow acoustic monitoring
Rainbow acoustic monitoring provides noninvasive and continuous measurement of respiration rate using an adhesive sensor with an integrated acoustic transducer that is applied to the patient's neck.[38][39] Researchers have evaluated acoustic respiration rate (RRa) and found the acceptable accuracy and significantly fewer false alarms than traditional respiration rate monitoring methods, end-tidal carbon dioxide (EtCO2) and impedance pneumography.[40]
SedLine brain function monitoring
In 2010, Masimo began offering brain function monitoring to measure the effects of anesthesia and sedation by monitoring both sides of the brain's electrical activity (EEG). Studies have shown this results in more individualized titration and improved care.[41]
Capnography and gas monitoring
In 2012, Masimo began offering ultra-compact mainstream and sidestream capnography as well as multigas analyzers for end-tidal carbon dioxide (CO2), nitrous oxide (N2O), oxygen (O2), and anesthetic agents, for use in the operating room, procedural sedation, and in intensive care units (ICU).[42] A multi-center study at Cincinnati Children's Hospital Medical Center, University Medical Center (Tucson, Arizona), and Children's Medical Center (Dallas), found that respiratory rate measured from noninvasive, acoustic monitoring had similar accuracy and precision as nasal capnography, the current standard of care when used in pediatric patients.[43]
Radius-7
On December 1, 2014, Masimo announced FDA 510(k) clearance of Radius-7 for the Root patient monitoring and connectivity platform, the first and only wearable, wireless monitor with Masimo’s rainbow SET technology, enabling early identification of clinical deterioration while offering patients continuous monitoring with freedom of movement. Radius-7 attaches to the patient’s arm or can be placed alongside the patient in their bed, allowing untethered monitoring. Studies have shown that patient mobility is a key factor in more rapid patient recovery.[44]
iSpO2 pulse oximeter for smart phones and tablets
iSpO2 pulse oximeter was awarded the Hot Product Award at the 2013 EMS Today Conference & Exposition.[45]
Supported Causes
Clinton Global Initiative
In 2012, Masimo embarked on its first Commitment to Action with the Clinton Global Initiative (CGI) to solve the global problem of maternal mortality and anemia. The $1 million, two-year project initially focuses on five villages in Liberia and Uganda – two countries where the epidemics of maternal mortality and anemia are among the worst.[46]
Patient Safety Movement Foundation
In 2013, Masimo founder, Chairman and CEO Joe Kiani, created the nonprofit Patient Safety Movement Foundation with a mission to eliminate the more than 200,000[47] preventable patient deaths that occur in U.S. hospitals each year. The foundation holds annual Patient Safety, Science & Technology Summits, featuring leaders from healthcare, industry, and government; former President Bill Clinton has served as the keynote speaker.
References
- ↑ Homepage "Error: no
|title=specified when using {{Cite web}}". masimo.com. http://masimocorp.gcs-web.com/static-files/b99fe298-4f62-4b3b-bb5a-de8532c99dea; Homepage. Retrieved 2017-07-27. - ↑ "Masimo Corporation: NASDAQ:MASI quotes & news - Google Finance". Finance.google.com. http://finance.google.com/finance?q=NASDAQ:MASI. Retrieved 2012-08-05.
- ↑ "iData Research, Inc. Research Reports". Marketresearch.com. http://www.marketresearch.com/vendors/viewVendor.asp?VendorID=3689. Retrieved 2012-08-05.
- ↑ "Masimo age". getnetworth.com. http://www.getnetworth.com/tag/masimo-age/. Retrieved 2014-10-09.
- ↑ "Masimo recognized as top 10 innovator in medical device industry". Medicalnewstoday.com. http://www.medicalnewstoday.com/articles/46369.php. Retrieved 2012-08-05.
- ↑ "Joe Kiani, CEO, Chairman and Founder of Masimo Corporation, rings the NASDAQ opening bell". Nasdaq.com. 2008-08-07. http://www.nasdaq.com/reference/200808/market_open_080708.stm.
- ↑ Badenhausen, K (2011-10-19). "The top 20 small public companies in America". Forbes.com. https://www.forbes.com/sites/kurtbadenhausen/2011/10/19/the-top-20-small-public-companies-in-america/.
- ↑ Badenhausen, K (2011-10-19). "#19 Masimo". Forbes.com. https://www.forbes.com/pictures/mli45gdjh/19-masimo.
- ↑ "Creator of non-invasive patient monitoring devices: Masimo Corporation founder and CEO Joe Kiani named Ernst & Young National Entrepreneur Of The Year 2012 Life Sciences Award Winner". PRNewswire.com. 2012-11-18. http://www.prnewswire.com/news-releases/creator-of-non-invasive-patient-monitoring-devices-masimo-corporation-founder-and-ceo-joe-kiani-named--ernst--young-national-entrepreneur-of-the-year-2012-life-sciences-award-winner-179843111.html.
- ↑ 10.0 10.1 Barker, SJ (2002). ""Motion-resistant" pulse oximetry: a comparison of old and new models". pp. 967–72. doi:10.1213/00000539-200210000-00033. https://www.ncbi.nlm.nih.gov/pubmed?term=barker%202002%20pulse%20oximetry%20masimo.
- ↑ Mardirossian, G; Schneider, RE (1992). "Limitations of pulse oximetry". Anesth Prog 39 (6): 194–6. PMID 8250340.
- ↑ Barker, SJ; Tremper, KK (1987). "Pulse oximetry: Applications and limitations". Int Anesthesiol Clin 25 (3): 155–75. doi:10.1097/00004311-198702530-00010. PMID 3323062.
- ↑ Malviya, S; Reynolds, PI; Voepel-Lewis, T; Siewert, M; Watson, D; Tait, AR; Tremper, K (2000). "False alarms and sensitivity of conventional pulse oximetry versus the SET technology in the pediatric postanesthesia care unit". Anesth Analg 90 (6): 1336–40. doi:10.1097/00000539-200006000-00013. PMID 10825316.
- ↑ Castillo, A; Deulofeut, R; Critz, A; Sola, A (2011). "Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 technology". Acta Paediatrica 100 (2): 188–92. doi:10.1111/j.1651-2227.2010.02001.x. PMID 20825604.
- ↑ Bizzarro, M; Ly, F; Katz, K; Shabanova, V; Ehrenkranz, R; Bhandari, V (2013). "Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit". J Perinatol 34: 33–8. doi:10.1038/jp.2013.122.
- ↑ De-Wahl, GA; Wennergren, M; Sandberg, K; Mellander, M; Bejlum, C; Inganas, L; Eriksson, M; Segerdahl, N et al. (2009). "Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns". Br Med J 338: a3037. doi:10.1136/bmj.a3037.
- ↑ Durbin, CG; Rostow, SK (2002). "More reliable oximetry reduces the frequency of arterial blood gas analysis and hastens oxygen weaning following cardiac surgery; a prospective randomize trial of the clinical impact of a new technology". Crit Care Med 30 (8): 1735–40. doi:10.1097/00003246-200208000-00010.
- ↑ Taenzer, AH; Pyke, JB; McGrath, SP; Blike, GT (2010). "Impact of pulse oximetry surveillance on rescue events and intensive care unit (ICU) transfers: A before-and-after concurrence study". Anesthesiology 112: 282–87. doi:10.1097/ALN.0b013e3181ca7a9b. PMID 20098128.
- ↑ Taenzer, AH; Blike, GT (2012). "Postoperative monitoring – the Dartmouth experience". APSF Spring-Summer Newsletter.
- ↑ Forget, P; Lois, F; De Kock, M (2010). "Goal-directed fluid management based on the pulse oximeter–derived pleth variability index reduces lactate levels and improves fluid management". Anesth Analg: 1. doi:10.1213/ANE.0b013e3181eb624f.
- ↑ Ewer, AK; Furmston, AT; Middleton, LJ; Deeks, J; Daniels, JP; Pattison, HM; Powell, R; Roberts, TE et al. (2012). "Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness". Health Technol Assess 16: 1–184. doi:10.3310/hta16020.
- ↑ "Masimo oximeters and neonatal sensors receive FDA 510(k) clearance with labeling for use in newborn screening for Critical Congenital Heart Disease (CCHD)" (Press release). PRNewswire.com. 2012-09-26.
- ↑ "IOFM technology adoption pack published". Ntac.nhs.uk. http://www.ntac.nhs.uk/NewsAndEvents/IOFM_Technology_Adoption_Pack_Published.aspx. Retrieved 2012-08-05.
- ↑ Vallet, B; Blanloeil, Y; Cholley, B; Orliaguet, G; Pierre, S; Tavernier, B (2012). "Strategy for perioperative vascular filling - guidelines for perioperative haemodynamic optimization" (Experts’ formalized recommendations, French Society of Anaesthesia and Intensive Care (SFAR), validation by the administrative council of SFAR on 19 October 2012).
- ↑ "Masimo Rainbow SET Pulse CO-Oximetry technology shown effective and efficient in detecting carbon monoxide poisoning in multiple clinical settings". Medicalnewstoday.com. http://www.medicalnewstoday.com/articles/59424.php. Retrieved 2012-08-05.
- ↑ "Anesthesiology News". Anesthesiology News. http://www.anesthesiologynews.com/ViewArticle.aspx?d=PRN&d_id=21&i=November+2010&i_id=679&a_id=16133. Retrieved 2012-08-05.
- ↑ Awada, WNFM; Maher, F (2013). "Reduction in red blood cell transfusions during neurosurgery with noninvasive and continuous hemoglobin monitoring". p. 51.
- ↑ Suner, S; Partridge, R; Sucov, A; Valente, J; Chee, K; Hughes, A; Jay, G (2008). "Non-invasive pulse co-oximetry screening in the emergency department identifies occult carbon monoxide toxicity". J Emerg Med 34 (4): 441–50. doi:10.1016/j.jemermed.2007.12.004. PMID 18226877.
- ↑ Roth, D; Schreiber, W; Herkner, H; Havel, C (2014). "Prevalence of carbon monoxide poisoning in patients presenting to a large emergency department". Int J Clin Pract 68 (10): 1239–45. doi:10.1111/ijcp.12432. PMID 24698635.
- ↑ Hampson, N (2012). "Noninvasive pulse co-oximetry expedites evaluation and management of patients with carbon monoxide poisoning". Am J Emerg Med 30 (9): 2021–4. doi:10.1016/j.ajem.2012.03.026.
- ↑ "Award winners | medical design excellence awards". Canontradeshows.com. 2005-10-22. http://www.canontradeshows.com/expo/awards/awards/index.php?catId=-1&year=2011&view=View. Retrieved 2012-08-05.
- ↑ "Noninvasive hemoglobin test (first ouchless & bloodless) makes world health organization’s (WHO) list of innovative medical technologies that address global health concerns and needs…". www.healthcarereview.com. 2010-08-31. http://www.healthcarereview.com/2010/08/noninvasive-hemoglobin-test-first-ouchless-bloodless-makes-world-health-organizations-who-list-of-innovative-medical-technologies-that-address-global-health-concerns-and-needs/.
- ↑ Ostrovsky, G (2007-10-17). "Masimo Patient SafetyNet". Medgadget.com. http://medgadget.com/archives/2007/10/masimo_patient_safetynet.html.
- ↑ "Geisel School of Medicine - Closer watch, fewer Intensive Care Unit (ICU) trips, Dartmouth anesthesiologists find". Dms.dartmouth.edu. 2010-03-05. http://dms.dartmouth.edu/news/2010/03/05_taenzer.shtml.
- ↑ "APSF Newsletter Spring-Summer 2012". Apsf.org. http://www.apsf.org/newsletters/html/2012/spring/index.htm. Retrieved 2012-08-05.
- ↑ "ECRI Institute announces winner of 4th annual health devices achievement award". Ecri.org. https://www.ecri.org/Press/Pages/Health_Devices_Award_Winner_2009.aspx. Retrieved 2012-08-05.
- ↑ Ostrovsky, G (2010-10-14). "Masimo to Release New Halo Index, Radical 7 Pulse Oximeter, Patient SafetyNet Monitoring System". Medgadget.com. http://www.medgadget.com/archives/2010/10/masimo_to_release_new_halo_index_radical_7_pulse_oximeter_patient_safetynet_monitoring_system.html.
- ↑ "FDA clears Masimo Rainbow SET Acoustic Respiration Rate Monitor | News | RT: for decision makers in respiratory care". Rtmagazine.com. Archived from the original on 2012-03-21. https://web.archive.org/web/20120321162436/http://www.rtmagazine.com/news/2009-12-01_01.asp. Retrieved 2012-08-05.
- ↑ Kumpula, JC; Rhonda, D; Harrison, RD (2010-10-18). "Accuracy of acoustic respiration rate monitoring in an acute nursing unit" (Abstract). ASA Abstracts. http://www.asaabstracts.com/strands/asaabstracts/abstract.htm;jsessionid=ED84A8C2F2FA0F72B892E720DA7F87A2?year=2010&index=15&absnum=1947.
- ↑ Goudra, B; Penugonda, L (2011). "Monitoring respiration in upper GI endoscopy anesthesia" (ASA Presentation). A246.
- ↑ Drover, DR; Lemmens, HJ; Pierce, ET; Plourde, G; Loyd, G; Ornstein, E; Prichep, LS; Chabot, RJ et al. (2002). "Patient state index: Titration of delivery and recovery from propofol, alfentanil, and nitrious oxide anesthesia". Anesthesiology 97 (1): 82–9. doi:10.1097/00000542-200207000-00012. PMID 12131107. http://journals.lww.com/anesthesiology/Fulltext/2002/07000/Patient_State_Index__Titration_of_Delivery_and.12.aspx.
- ↑ "Masimo enters noninvasive multigas monitoring, including capnography, with acquisition of PHASEIN". Medcitynews.com. 2012-08-01. http://medcitynews.com/2012/08/masimo-enters-noninvasive-multigas-monitoring-including-capnography-with-acquisition-of-phasein/. Retrieved 2012-08-05.
- ↑ Patino, M; Redford, DT; Quigley, TW; Mahmoud, M; Kurth, CD; Szmuk, P (Dec 2013). "Accuracy of acoustic respiration rate monitoring in pediatric patients". Paediatr Anaesth 23 (12): 1166–73. doi:10.1111/pan.12254. PMID 24033591.
- ↑ Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation Vol 91, Issue 4, PP 536–542, April 2010
- ↑ "Masimo Patient Monitoring Devices Earn Awards From JEMS". http://www.rtmagazine.com/2013/05/masimo-patient-monitoring-devices-earn-awards-from-jems/. Retrieved 2014-07-10.
- ↑ "Masimo announces $1 million commitment to action with the Clinton Global Initiative to solve maternal mortality & anemia". StarAfrica.com. 2012-09-25. http://www.starafrica.com/en/news/detail-news/view/masimo-announces-1-million-commitment-t-254163.html.
- ↑ JT, James (2013), "A new, evidence-based estimate of patient harms associated with hospital care", Journal of Patient Safety 9 (3): 122–8, PMID 23860193
Resources