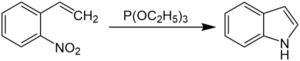Chemistry:Cadogan-Sundberg indole synthesis
| Cadogan-Sundberg indole synthesis | |
|---|---|
| Named after | John Cadogan Richard J. Sundberg |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000509 |
The Cadogan-Sundberg indole synthesis, or simply Cadogan indole synthesis, is a name reaction in organic chemistry that allows for the generation of indoles from o-nitrostyrenes with the use of trialkyl phosphites, such as triethyl phosphite.[1][2][3][4][5][6][7]
Mechanism
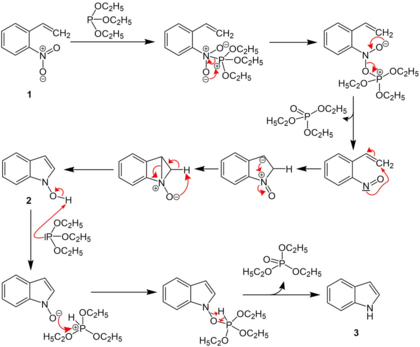
o-nitrostyrene first reacts with triethyl phosphite, and the nitro group is converted to a nitroso group. The nitroso group then reacts with the alkene, and N-hydroxylindole is formed, which reacts again with triethyl phosphite to form the indole.[1][2][3][4]
Application
The Cadogan-Sundberg indole synthesis has been used as an intermediate step in the total synthesis of Tjipanazole E,[8] transforming 2-[trans-2-[5-Chloro-2-nitrophenyl)vinyl]-5-chloro-1H-indole to 5,5’-Dichloro-2,2’-biindole.
References
- ↑ 1.0 1.1 Cadogan, John Ivan George; Mackie, R. K. (1974). "Tervalent phosphorus compounds in organic synthesis". Chemical Society Reviews 3 (1): 87-137. doi:10.1039/CS9740300087.
- ↑ 2.0 2.1 Wang, Zerong (15 September 2010). Comprehensive Organic Name Reactions and Reagents. doi:10.1002/9780470638859.conrr128. ISBN 9780470638859.
- ↑ 3.0 3.1 Sundberg, Richard J.; Yamazaki, Toshio (1 February 1967). "Rearrangements and ring expansions during the deoxygenation of β,β-disubstituted o-nitrostyrenes". The Journal of Organic Chemistry 32 (2): 290-294. doi:10.1021/jo01288a009.
- ↑ 4.0 4.1 Sundberg, Richard J. (1 November 1965). "Deoxygenation of Nitro Groups by Trivalent Phosphorus. Indoles from o-Nitrostyrenes". The Journal of Organic Chemistry 30 (11): 3604-3610. doi:10.1021/jo01022a006.
- ↑ Gribble, Gordon W. (17 June 2016). "Cadogan–Sundberg Indole Synthesis". Indole Ring Synthesis: From Natural Products to Drug Discovery. doi:10.1002/9781118695692.ch26. ISBN 9781118695692.
- ↑ Majgier-Baranowska, Helena; Williams, John D.; Li, Bing; Peet, Norton P. (1 February 1967). "Studies on the mechanism of the Cadogan–Sundberg indole synthesis". Tetrahedron Letters 53 (35): 4785-4788. doi:10.1016/j.tetlet.2012.06.146.
- ↑ Li, Jie Jack (4 January 2014). "Cadogan–Sundberg indole synthesis". Indole Ring Synthesis: From Natural Products to Drug Discovery. Springer. p. 102-103. doi:10.1007/978-3-319-03979-4_49. ISBN 978-3-319-03979-4.
- ↑ Kuethe, Jeffrey T.; Wong, Audrey; Davies, Iaan W. (3 September 2003). "Effective Strategy for the Preparation of Indolocarbazole Aglycons and Glycosides: Total Synthesis of Tjipanazoles B, D, E, and I". Organic Letters 5 (20): 3721–3723. doi:10.1021/ol035418r.