Chemistry:Ferrario-Ackermann reaction
From HandWiki
Revision as of 16:59, 26 May 2022 by imported>John Stpola (url)
| Ferrario-Ackermann reaction | |
|---|---|
| Named after | M. E. Ferrario Fritz Ackermann |
| Reaction type | Ring forming reaction |
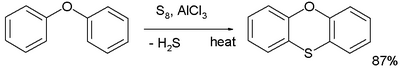
In organic chemistry, the Ferrario-Ackermann reaction or simply the Ferrario reaction is a name reaction that allow for the generation of phenoxanthiine from diphenyl ether and sulfur in the presence of aluminum chloride catalyst.[1][2][3][4][5][6]
References
- ↑ Ferrario, E. (January 1911). "Preparation of phenoxathiin from diphenyl ether and sulfur". Bulletin de la Société Chimique de France 9 (4): 536-537. https://gallica.bnf.fr/ark:/12148/bpt6k282044r.image.f540.langEN.
- ↑ Fritz Ackermann, "Verfahren zur Darstellung von Phenoxthin und dessen Derivaten", Germany patent 234743, published 20 May 1911
- ↑ Deasy, Clara L. (1 April 1943). "The Chemistry of Phenoxathiin and its Derivatives". Chemical Reviews 32 (2): 173-194. doi:10.1021/cr60102a001.
- ↑ Suter, C. M.; Maxwell, Charles E.. "Phenoxthin [Phenoxathiin"]. Organic Syntheses 18: 64. doi:10.15227/orgsyn.018.0064. http://www.orgsyn.org/demo.aspx?prep=CV2P0485.; Collective Volume, 2, pp. 485
- ↑ Al-Araji, Suad M.; Mohamad, Ayad Ahmed (2 June 201). "Synthesis of New Pyrazoline - Phenoxathiin Derivatives". Baghdad Science Journal 10 (2): 405-419. doi:10.21123/bsj.2013.10.2.405-419. https://bsj.uobaghdad.edu.iq/index.php/BSJ/article/view/1464.
- ↑ Suter, C. M.; Green, Frank O. (1 December 1937). "Phenoxthin. II. Extension of the Ferrario Reaction". Journal of the American Chemical Society 59 (12): 2578–2580. doi:10.1021/ja01291a030.