Chemistry:Cross electrophile coupling
Cross electrophile coupling is a type of cross-coupling reaction that occurs between two electrophiles often catalyzed by transition metal catalyst(s). Unlike conventional cross-coupling reactions of an electrophile with an organometallic reagent,[1] the coupling partners in cross electrophile coupling reactions are both electrophiles.[2] Generally, additional reductant to regenerate active catalyst is needed in this reaction.
Cross selectivity
In contrast to the reductive dimerization of electrophiles, which has long been known (Wurtz reaction, Ullman reaction, etc.),[3][4] limited methods exist for achieving cross selectivity. This selectivity issue comes from the similarity of two reagents participate in the reaction. There are several strategies to address this problem.
Using excess of one reagent
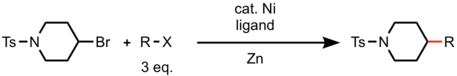
Using excess amount of one reagent can provide synthetically useful yields of cross-coupled product when the starting materials have similar reactivity. (e.g. coupling between two alkyl halides). Using this strategy, Gong reported cross-couple reaction between two alkyl halides with Ni catalyst using zinc as reductant.[5] Dimerization of the more reactive alkyl halide was the major byproduct in these reactions.
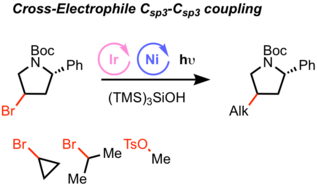
Recently, alkyl-alkyl (Csp3-Csp3) cross electrophile coupling using metallaphotoredox catalysis was achieved by MacMillan group.[6] In this case, small alkyl electrophiles were used excess amount. Tris(trimethylsilyl)silanol can be employed as a halogen abstractor in combination with photoredox and Ni catalysis.
Sequential oxidative addition
In cases where two electrophiles have different chemical reactivity, this difference can be used to achieve high selectivity for cross product.
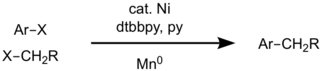
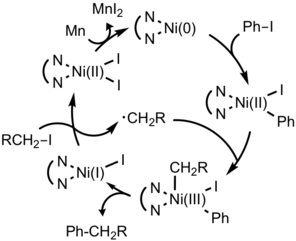
For example, aryl halide and alkyl halide can be differentiated by the reactivity trends of two electrophiles whether it prefers heterolytic or homolytic pathway.[7] [8] Through detailed mechanistic studies, the origin of high selectivity was revealed : Aryl halides do faster oxidative addition to Ni(0) center than alkyl halides, however, alkyl halides form radicals when it react with nickel center. The catalysis begins with oxidative addition of an aryl halide to Ni(0). The resulting Ni(II) species reacts with an alkyl radical generated from alkyl halides to form diorganonickel(III) intermediate which can undergo reductive elimination of the cross coupled product. Reduction of Ni(II) to Ni(0) by Mn closes the catalytic cycle.
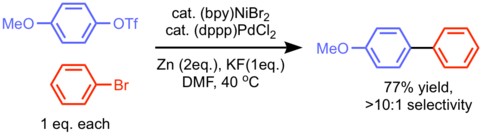
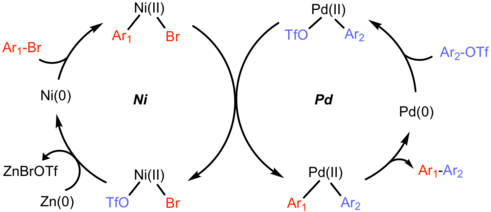
In 2015, reductive cross coupling of two different aryl (pseudo)halide was achieved by Weix group. Use of two different metals (Ni and Pd) was essential for the observed cross-selectivity of chemically similar two reactants. Aryl triflates generally prefer oxidative addition to the electron-rich Pd(0) catalyst whereas Ni(0) selectively reacts with aryl bromides.[9]
Transmetallation of the aryl-nickel(II) onto the Aryl-palladium(II) forms a Pd(II)Ar1Ar2 species followed by reductive elimination to give product. Finally, reduction of Ni(II) by additive zinc regenerates Ni(0).
References
- ↑ Hartwig, John Frederick (2010). Organotransition metal chemistry : from bonding to catalysis. University Science Books. ISBN 9781891389535. OCLC 781082054.
- ↑ "Cross-electrophile coupling: principles of reactivity and selectivity". The Journal of Organic Chemistry 79 (11): 4793–8. June 2014. doi:10.1021/jo500507s. PMID 24820397.
- ↑ Hassan, Jwanro; Sevignon, Marc; Gozzi, Christel; Schulz, Emmanuelle; Lemaire, Marc (2002). "Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction". Chemical Reviews 102 (5): 1369–1469. doi:10.1021/cr000664r. PMID 11996540.
- ↑ "Ueber eine neue Klasse organischer Radicale" (in en). Justus Liebigs Annalen der Chemie 96 (3): 364–375. 1855. doi:10.1002/jlac.18550960310. ISSN 1099-0690. https://zenodo.org/record/1427074.
- ↑ "Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides". Organic Letters 13 (8): 2138–41. April 2011. doi:10.1021/ol200617f. PMID 21434609.
- ↑ "3 Coupling of Aliphatic Bromides". Journal of the American Chemical Society 140 (50): 17433–17438. December 2018. doi:10.1021/jacs.8b12025. PMID 30516995.
- ↑ "Replacing conventional carbon nucleophiles with electrophiles: nickel-catalyzed reductive alkylation of aryl bromides and chlorides". Journal of the American Chemical Society 134 (14): 6146–59. April 2012. doi:10.1021/ja301769r. PMID 22463689.
- ↑ "Mechanism and selectivity in nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides". Journal of the American Chemical Society 135 (43): 16192–7. October 2013. doi:10.1021/ja407589e. PMID 23952217.
- ↑ "Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates". Nature 524 (7566): 454–7. August 2015. doi:10.1038/nature14676. PMID 26280337. Bibcode: 2015Natur.524..454A.
 |