Chemistry:Leuckart thiophenol reaction
From HandWiki
Revision as of 20:44, 30 June 2021 by imported>WikiG (url)
| Leuckart thiophenol reaction | |
|---|---|
| Named after | Rudolf Leuckart |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | leuckart-thiophenol-reaction |
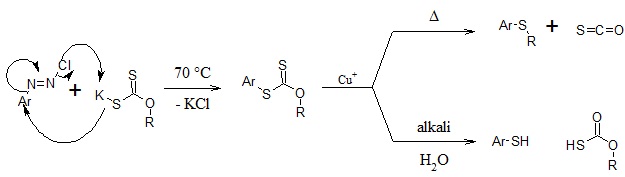
The Leuckart thiophenol reaction is the decomposition of a diazoxanthate, by gentle warming in a slightly acidic cuprous medium, to its corresponding aryl xanthates which give aryl thiols on alkaline hydrolysis and aryl thioethers on further warming.[1]
This reaction was first reported by Rudolf Leuckart in 1890.[2][3][4]
References
- ↑ Kazem-Rostami, Masoud; Khazaei, Ardeshir; Moosavi-Zare, Ahmad; Bayat, Mohammad; Saednia, Shahnaz (2012). "synthesis of thiophenols from related triazenes". Synlett 23 (13): 1893–1896. doi:10.1055/s-0032-1316557.
- ↑ Leuckart, Rudolf (30 December 1889). "Eine neue Methode zur Darstellung aromatischer Mercaptane". Journal für Praktische Chemie 41 (1): 179–224. doi:10.1002/prac.18900410114. https://zenodo.org/record/1427950.
- ↑ Fukushima, D. K.; Tarbell, D. S. (1947). "m-THIOCRESOL". Organic Syntheses 27: 81. doi:10.15227/orgsyn.027.0081.
- ↑ Merck Index 14th Ed.
 |