Chemistry:Polyring forming processes
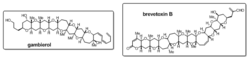
Polycyclic natural products such as marine toxin gambierol and brevetoxin B (Fig. 1) are intriguing targets in organic synthesis. Polyring forming processes are applied to the total synthesis of these polycyclic molecules.[1] Short sequences of reactions are used in an iterative fashion to build the successive ring structures.
Brevetoxin B synthesis
Brevetoxin B was first synthesized by K. C. Nicolaou and co-workers in 1995.[2] Along the campaign towards completion of the total synthesis of brevetoxin B, polyring forming processes that consists of iterative epoxide ring-opening reactions was used to construct the ether linkages in one fragment of brevetoxin B (Fig. 2).
Polypyran synthesis
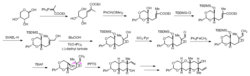
Mori and co-workers have developed a short iterative strategy for the synthesis of polypyran domains in natural products (Fig. 3).[3] This strategy is also based on epoxide ring-opening reactions and consists totally 6 steps in each iterative cycle. The epoxide is installed using oxiranyl anions generated from reacting epoxide B with strong base such as n-butyllithium. Treating the product C with acid afforded the desired ring product which can be further converted to the next precursor D in four steps.
References
- ↑ Marmsater, F. P.; West, F. G. Chem. Eur. J. 2002, 8, 4347.
- ↑ Nicolaou, K. C.; Theodorakis, E. A.; Rutjes, F. P. J. T.; Sato, M.; Tiebes, J.; Xiao, X.-Y.; Hwang, C.-K.; Duggan, M. E.; Yang, Z.; Couladouros, E. A.; Sato, F.; Shin, J.; He, H.-M.; Bleckman, T. J. Am. Chem. Soc. 1995, 117, 10239.
- ↑ Mori, Y.; Yaegeshi, K.; Furukawa, H. J. Am. Chem. Soc. 1996, 118, 8158.
 |