Iodine test
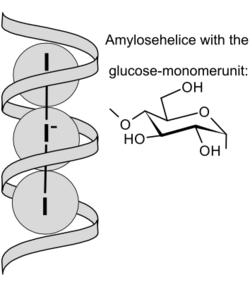 Schematic view of I3− ions embedded in amylose helix | |
| Classification | Colorimetric method |
|---|---|
| Analytes | Starch |
The iodine test is used to test for the presence of starch. Starch turns into an intense "blue-black" colour upon addition of aqueous solutions of the triiodide anion, due to the formation of an intermolecular charge-transfer complex. In the absence of starch, the brown color of the aqueous solution remains. This interaction between starch and triiodide is also the basis for iodometry.
Principles
To produce the triiodide anion (I3−), elemental iodine is dissolved in an aqueous solution of potassium iodide. The resulting complex with starch produces an intense "blue-black" colour. The intensity of the colour decreases with increasing temperature and with the presence of water-miscible organic solvents such as ethanol . The test cannot be performed at very low pH due to the hydrolysis of the starch under these conditions.[1] It is now thought that the iodine-iodide mixture combines with the starch to form an infinite polyiodide homopolymer. This was rationalized through single crystal x-ray crystallography and comparative Raman spectroscopy.[2]
Starch as an indicator
Starch is often used in chemistry as an indicator for redox titrations where triiodide is present. Starch forms a very dark blue-black complex with triiodide which can be made by mixing iodine with iodide (often from potassium iodide). However, the complex is not formed if only iodine or only iodide (I−) is present. The colour of the starch complex is so deep, that it can be detected visually when the concentration of the iodine is as low as 0.00002 M at 20 °C.[3] During iodine titrations, concentrated iodine solutions must be reacted with some titrant, often thiosulfate, in order to remove most of the iodine before the starch is added. This is due to the insolubility of the starch-triiodide complex which may prevent some of the iodine reacting with the titrant. Close to the end-point, the starch is added, and the titration process is resumed taking into account the amount of thiosulfate added before adding the starch.
The color change can be used to detect moisture or perspiration, as in the Minor test or starch–iodine test.
See also
- Lugol's iodine
- Counterfeit banknote detection pen
References
- ↑ "Iodine Test for Starch". http://brilliantbiologystudent.weebly.com/iodine-test-for-starch.html. Retrieved 2015-12-01.
- ↑ Madhu, Sheri; Evans, Hayden A.; Doan-Nguyen, Vicky V. T.; Labram, John G.; Wu, Guang; Chabinyc, Michael L.; Seshadri, Ram; Wudl, Fred (4 July 2016). "Infinite Polyiodide Chains in the Pyrroloperylene-Iodine Complex: Insights into the Starch-Iodine and Perylene-Iodine Complexes". Angewandte Chemie International Edition 55 (28): 8032–8035. doi:10.1002/anie.201601585. PMID 27239781.
- ↑ Bertrand, Gary. "Iodine Clock Reaction". http://web.mst.edu/~gbert/IClock/discussion.htm.
Further reading
- Vogel's Textbook of Quantitative Chemical Analysis, 5th edition.
External links
- How does starch indicate iodine? General Chemistry Online
- Iodine test at Braukaiser
- Titrations.info: Potentiometric titration--Solutions used in iodometric titrations

