Medicine:Hyperphosphatasia with mental retardation syndrome
| Hyperphosphatasia with mental retardation syndrome | |
|---|---|
| Other names | Mabry syndrome |
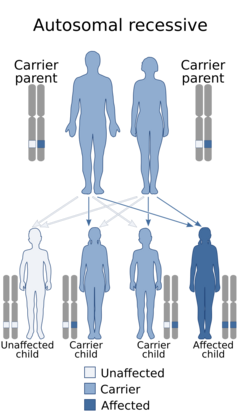 | |
| This condition is inherited in an autosomal recessive manner | |
Hyperphosphatasia with mental retardation syndrome, HPMRS,[1] also known as Mabry syndrome,[2] has been described in patients recruited on four continents world-wide.[3] Mabry syndrome was confirmed[4] to represent an autosomal recessive syndrome characterized by severe mental retardation, considerably elevated serum levels of alkaline phosphatase, hypoplastic terminal phalanges, and distinct facial features that include: hypertelorism, a broad nasal bridge and a rectangular face.
Pathogenesis
While many cases of HPMRS are caused by mutations in the PIGV gene,[5] there may be genetic heterogeneity in the spectrum of Mabry syndrome as a whole.[2] PIGV is a member of the molecular pathway that synthesizes the glycosylphosphatidylinositol anchor.[6] The loss in PIGV activity results in a reduced anchoring of alkaline phosphatase to the surface membrane and an elevated alkaline phosphatase activity in the serum.[citation needed]
Diagnosis
The clinical diagnosis can be established if the patient has repeatedly elevated levels of alkaline phosphatase activity in the blood serum and exhibits intellectual disability. Supportive for the clinical diagnosis are epilepsies, brachydactyly and a characteristic facial gestalt, which can also be assessed by means of AI.[7] The clinical diagnosis can be confirmed by molecular testing such as exome sequencing.
Treatment
So far, no effective treatment is available for HPMRS. A mouse model that mirrors the human phenotype has been engineered by CRISPR technology and is available for compound screening.[8]
References
- ↑ "Familial hyperphosphatase with mental retardation, seizures, and neurologic deficits". The Journal of Pediatrics 77 (1): 74–85. July 1970. doi:10.1016/s0022-3476(70)80047-6. PMID 5465362.
- ↑ 2.0 2.1 "Hyperphosphatasia with seizures, neurologic deficit, and characteristic facial features: Five new patients with Mabry syndrome". American Journal of Medical Genetics. Part A 152A (7): 1661–1669. July 2010. doi:10.1002/ajmg.a.33438. PMID 20578257.
- ↑ "Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder?". Pediatric Neurology 34 (4): 303–307. 2006. doi:10.1016/j.pediatrneurol.2005.08.020. PMID 16638507.
- ↑ "Hyperphosphatasia with mental retardation, brachytelephalangy, and a distinct facial gestalt: Delineation of a recognizable syndrome". European Journal of Medical Genetics 53 (2): 85–88. 2010. doi:10.1016/j.ejmg.2010.01.002. PMID 20080219.
- ↑ "Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome". Nature Genetics 42 (10): 827–829. October 2010. doi:10.1038/ng.653. PMID 20802478.
- ↑ "PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol". The Journal of Biological Chemistry 280 (10): 9489–9497. March 2005. doi:10.1074/jbc.M413867200. PMID 15623507.
- ↑ "Characterization of glycosylphosphatidylinositol biosynthesis defects by clinical features, flow cytometry, and automated image analysis". Genome Medicine 10 (1): 3. January 2018. doi:10.1186/s13073-017-0510-5. PMID 29310717.
- ↑ "A CRISPR-Cas9-engineered mouse model for GPI-anchor deficiency mirrors human phenotypes and exhibits hippocampal synaptic dysfunctions". Proceedings of the National Academy of Sciences of the United States of America 118 (2): e2014481118. January 2021. doi:10.1073/pnas.2014481118. PMID 33402532. Bibcode: 2021PNAS..11814481R.
External links
| Classification | |
|---|---|
| External resources |
 |

