Biology:Ameloblast
| Ameloblast | |
|---|---|
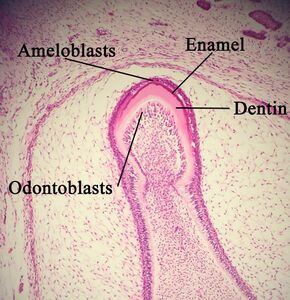 A developing tooth with ameloblasts marked. | |
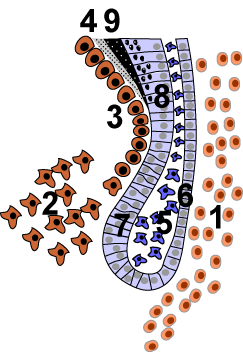 The cervical loop area: (1) dental follicle cells, (2) dental mesenchyme, (3) Odontoblasts, (4) Dentin, (5) stellate reticulum, (6) outer enamel epithelium, (7)inner enamel epithelium, (8) ameloblasts, (9) enamel. | |
| Details | |
| Identifiers | |
| Latin | ameloblastus |
| Anatomical terms of microanatomy | |
Ameloblasts are cells present only during tooth development that deposit tooth enamel, which is the hard outermost layer of the tooth forming the surface of the crown.
Structure
Each ameloblast is a columnar cell approximately 4 micrometers in diameter, 40 micrometers in length and is hexagonal in cross section. The secretory end of the ameloblast ends in a six-sided pyramid-like projection known as the Tomes' process. The angulation of the Tomes' process is significant in the orientation of enamel rods, the basic unit of tooth enamel. Distal terminal bars are junctional complexes that separate the Tomes' processes from ameloblast proper.
Development
Ameloblasts are derived from oral epithelium tissue of ectodermal origin. Their differentiation from preameloblasts (whose origin is from inner enamel epithelium) is a result of signaling from the ectomesenchymal cells of the dental papilla. Initially the preameloblasts will differentiate into presecretory ameloblasts and then into secretory ameloblasts which lay down the tooth enamel. The differentiation from preameloblasts to ameloblasts occurs during the first stage of amelogenesis, called the pre-secretory (or inductive) phase. [1]
The ameloblasts will only become fully functional after the first layer of dentin (predentin) has been formed by odontoblasts. The cells are part of the reduced enamel epithelium after enamel maturation and then subsequently undergo apoptosis before or after tooth eruption.[2][3]: 103 These stages occur during the third and final stage of amelogenesis, called the maturation phase.
There are various factors which can affect the differentiation and development of ameloblasts, causing abnormalities to form within the tooth structure. One example is the BMP (bone morphogenetic protein,) which has an important role in ameloblast differentiation. When Follistatin, a BMP inhibitor, is over expressed in the epithelium of developing teeth, the ameloblasts do not differentiate and no enamel forms. Another example includes the conditional deletion of Dicer-1 in the epithelium of developing teeth may cause impaired differentiation of ameloblasts which results in deficient enamel formation. [2]
Life cycle
The life cycle of ameloblasts[3]: 153 consists of six stages:
- Morphogenic stage
- Organizing stage
- Formative (secretory) stage (Tomes' processes appear)
- Maturative stages
- Protective stage
- Desmolytic stage

Ameloblast life cycle & Amelogenesis
The murine ALC (ameloblast like cell) cell line is of ameloblastic origin.[4]
1. Morphogenic Stage
In this morphogenic stage, the morphology of the cells are short, columnar with large oval nuclei. The golgi apparatus and centrioles are located in the proximal end of the ameloblasts, and mitochondria are dispersed throughout the cytoplasm.
2. Organizing Stage
In this stage, the ameloblast cells become longer and the nucleus migrates towards the proximal end. In contrast to this, golgi apparatus and centrioles migrate towards the distal end. This change is referred to as "REVERSAL OF POLARITY". During this stage, the odontoblasts start laying down dentin.
Reversal of Nutrition - as long as the ameloblasts are in contact with the dental papilla, they receive nutrient material from the blood vessels of the tissue, but due to formation of this dentin the original source of nutrition is cut off and the ameloblasts are supplied by capillaries penetrating the outer enamel epithelium. This change in nutrition source is referred to as "reversal of nutrition".
3. Formative Stage
In this stage, formation of enamel matrix begins. During the formation of enamel matrix, the ameloblasts retain approximately the same length.
4. Maturative Stage
After the formation of enamel matrix, mineralisation of enamel takes place which is known as maturation. During this stage, the ameloblasts are slightly reduced in length. The stratum intermedium cells lose their cuboidal shape and assumed to be as spindle shape. During this stage, ameloblasts also exhibit microvilli at their distal extremities.
5. Protective Stage
In this stage, enamel is completely developed and fully calcified. Now the cell layers form a stratified epithelial covering of enamel, which is known as reduced enamel epithelium. This reduced enamel epithelium protects the mature enamel.
6. Desmolytic Stage
In this stage, the reduced enamel epithelium proliferates and induce atrophy. The reduced enamel epithelium releases enzymes which destroy the connective tissue, in a process known as desmolysis.
Function
Ameloblasts are cells which secrete the enamel proteins enamelin and amelogenin which will later mineralize to form enamel, the hardest substance in the human body.[5] Ameloblasts control ionic and organic compositions of enamel. It is theorized that a circadian clock (24-hour) probably regulates enamel production on a daily cycle by the ameloblasts (similar to osteoblasts in production of bone tissue).[6] Ameloblasts adjust their secretory and resorptive activities to maintain favorable conditions for biomineralization.[3]: 147
Clinical significance
These cells are sensitive to their environment. One common example is illustrated by the neonatal line, a pronounced incremental line of Retzius found in the primary teeth and in the larger cusps of the permanent first molars, showing a disruption in enamel production when the person is born.[7] High fevers in childhood are also an example of bodily stressors causing interruptions in enamel production.
Another possible example of this sensitivity (stress response pathway activation) may be the development of dental fluorosis after childhood exposure (between the ages of 2 and 8 years old) to excess consumption of fluoride, an elemental agent used to increase enamel hardness and as a result, prevent dental caries.[8]
See also
- Ameloblastin
- Ameloblastoma
- Amelogenesis imperfecta
- Dentin
- Enamel
- Odontoblast
- Tooth development
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body
References
- ↑ "Ameloblast differentiation in the human developing tooth: effects of extracellular matrices". Matrix Biology 29 (5): 411–9. June 2010. doi:10.1016/j.matbio.2010.03.001. PMID 20211728.
- ↑ 2.0 2.1 Huang, George T.-J.; Thesleff, Irma (2013). Stem cells in craniofacial development and regeneration. Hoboken, New Jersey: Wiley-Blackwell. ISBN 9781118498118. OCLC 809365748.
- ↑ 3.0 3.1 3.2 Nanci, Antonio (2012). Ten Cate's oral histology : development, structure, and function. (Eighth ed.). Mosby. ISBN 978-0-323-24207-3.
- ↑ "Differentiation of an ameloblast-lineage cell line (ALC) is induced by Sonic hedgehog signaling". Biochemical and Biophysical Research Communications 353 (2): 405–11. February 2007. doi:10.1016/j.bbrc.2006.12.053. PMID 17188245.
- ↑ "Localization and quantitative co-localization of enamelin with amelogenin". Journal of Structural Biology 183 (2): 239–49. August 2013. doi:10.1016/j.jsb.2013.03.014. PMID 23563189.
- ↑ "Incremental lines in mouse molar enamel". Archives of Oral Biology 58 (10): 1443–9. October 2013. doi:10.1016/j.archoralbio.2013.06.013. PMID 23845754.
- ↑ Illustrated Dental Embryology, Histology, and Anatomy, Bath-Balogh and Fehrenbach, Elsevier, 2011, page 151
- ↑ "Stress response pathways in ameloblasts: implications for amelogenesis and dental fluorosis". Cells 1 (3): 631–45. September 2012. doi:10.3390/cells1030631. PMID 23745169.
External links
- "Regulation of dental enamel shape and hardness". Journal of Dental Research 89 (10): 1024–38. October 2010. doi:10.1177/0022034510375829. PMID 20675598.
 |
