Biology:Amniotic fluid
| Amniotic fluid | |
|---|---|
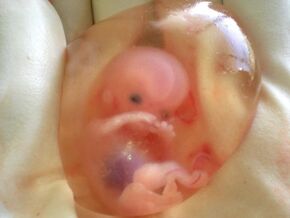 Ten-week-old human fetus surrounded by amniotic fluid within the amniotic sac | |
| Anatomical terminology |

The amniotic fluid is the protective liquid contained by the amniotic sac of a gravid amniote. This fluid serves as a cushion for the growing fetus, but also serves to facilitate the exchange of nutrients, water, and biochemical products between mother and fetus.
Colloquially, the amniotic fluid is commonly called water or waters (Latin liquor amnii).
Development
Amniotic fluid is present from the formation of the gestational sac. Amniotic fluid is in the amniotic sac. It is generated from maternal plasma, and passes through the fetal membranes by osmotic and hydrostatic forces. When fetal kidneys begin to function around week 16, fetal urine also contributes to the fluid.[1] In earlier times, it was believed that the amniotic fluid was composed entirely of excreted fetal urine.
The fluid is absorbed through the fetal tissue and skin.[2] After 22 to 25 week of pregnancy, keratinization of an embryo's skin occurs. When this process completes around the 25th week,[2] the fluid is primarily absorbed by the fetal gut for the remainder of gestation.[1]
Contents
At first, amniotic fluid is mainly water with electrolytes. By about the 12–14th week the liquid also contains proteins, carbohydrates, lipids and phospholipids, urea, and extracellular matrix (ECM) components including collagens and glycosaminoglycans, including hyaluronic acid and chondroitin sulfate, all of which aid in the growth of the fetus.
Volume
The volume of amniotic fluid changes with the growth of the fetus. From the tenth to the 20th week, it increases from 25 to 400 millilitres (0.88 to 14.08 imp fl oz; 0.85 to 13.53 US fl oz) approximately.[3] Approximately in the 10th–11th week, the breathing and swallowing of the fetus slightly decrease the amount of fluid. Neither urination nor swallowing contributes significantly to fluid quantity changes until the 25th week, when keratinization of skin is complete; then the relationship between fluid and fetal growth stops. It reaches a plateau of 800 millilitres (28 imp fl oz; 27 US fl oz) by the 28-week gestational age. The amount of fluid declines to roughly 400 millilitres (14 imp fl oz; 14 US fl oz) at 42 weeks.[3] Some sources indicate about 500 to 1,000 millilitres (18 to 35 imp fl oz; 17 to 34 US fl oz) of amniotic fluid is present at birth.[1][4]
Rupture of membranes
The forewaters are released when the amnion ruptures. This is commonly known as "water breaking." When this occurs during labour at term, it is known as "spontaneous rupture of membranes". If the rupture precedes labour at term, however, it is referred to as "pre-labour rupture of membranes." Spontaneous rupture of membranes before term is referred to as "premature rupture of membranes." The majority of the hindwaters remain inside the womb until the baby is born. Artificial rupture of membrane (ARM), a manual rupture of the amniotic sac, can also be performed to release the fluid if the amnion has not spontaneously ruptured.[5]
Function
Swallowed amniotic fluid (in later stages of development) creates urine and contributes to the formation of meconium. Amniotic fluid protects the developing fetus by cushioning against blows to the mother's abdomen, allowing for easier fetal movement, and promoting muscular/skeletal development. Amniotic fluid swallowed by the fetus helps in the formation of the gastrointestinal tract. It also protects the fetus from mechanical jerks and shocks. The fetus, which develops within a fluid-filled amniotic sac, relies on the placenta for respiratory gas exchange rather than the lungs. While not involved in fetal oxygenation, fetal breathing movements (FBM) nevertheless have an important role in lung growth and in the development of respiratory muscles and neural regulation.[6] FBM are regulated differently in many respects than postnatal respiration, which results from the unique intrauterine environment. At birth, the transition to continuous postnatal respiration involves a fall in temperature, gaseous distention of the lungs, activation of the Hering-Breuer reflex, and functional connectivity of afferent O2 chemoreceptor activity with respiratory motoneurons and arousal centers.[7]
Clinical significance
Collection
Amniotic fluid is removed from the mother by an amniocentesis procedure, where a long needle is inserted through the abdomen into the amniotic sac, using ultrasound guidance such that the fetus is not harmed. Amniocentesis is a low-risk procedure, with a risk of pregnancy loss between 1 in 1,500 – 1 in 700 procedures. Amniocentesis can be performed to obtain diagnostic genetic information, evaluate for intrauterine infection, or, rarely, to assess for fetal lung maturity if early delivery is required. If warranted, fluid is collected between 16 and 42 weeks of fetal development. The amount of fluid removed depends on the indication for the procedure and the testing that will be performed on the fluid.
Analysis
Analysis of amniotic fluid can reveal many aspects of the baby's genetic health as well as the age and viability of the fetus. This is because the fluid contains metabolic wastes and compounds used in assessing fetal age and lung maturity, but amniotic fluid also contains fetal cells, which can be examined for genetic defects.
Amniotic fluid normally has a pH of 7.0 to 7.5.[8] Because pH in the upper vagina is normally acidic (pH 3.8–4.5), a vaginal pH test showing a pH of more than 4.5 strengthens a suspicion of rupture of membranes in case of clear vaginal discharge in pregnancy.[8] Other tests for detecting amniotic fluid mainly include nitrazine paper test and fern test.[9] One main test that is performed on amniotic fluid is the L/S ratio test (lecithin/sphingomyelin). This test is used to determine fetal lung maturity. Both lecithin and sphingomyelin are lung surfactants that are present in increasing amounts in the maturing fetus, though past week 33, sphingomyelin levels remain relatively constant. Measuring a ratio of L/S of 2:1 or greater indicates that the fetus can be safely delivered, with functioning lungs.
Complications related to amniotic fluid
Too little amniotic fluid is called oligohydramnios. In a minority of cases, it can be a cause of problems for the mother and baby. These include contracture of the limbs, clubbing of the feet and hands, and also a life-threatening condition called hypoplastic lungs. The Potter sequence refers to a constellation of findings related to insufficient amniotic fluid.
The opposite of oligohydramnios is polyhydramnios, an excess volume of amniotic fluid in the amniotic sac.
Amniotic fluid embolism is a rare but very often fatal condition for both mother and child.
Medical applications
It is being used in some surgeries of the outside of the eye.[10] It is also being studied for some orthopaedic conditions.[11][12]
Stem cell research
Recent studies show that amniotic fluid contains a considerable quantity of stem cells.[13] These amniotic stem cells[14][15] are pluripotent and able to differentiate into various tissues, which may be useful for future human application.[16][17][18] Some researchers have found that amniotic fluid is also a plentiful source of non-embryonic stem cells.[19] These cells have demonstrated the ability to differentiate into many different cell-types, including brain, liver and bone.
It is possible to conserve the stem cells extracted from amniotic fluid in private stem cell banks.
See also
- Liquid breathing
- Potter's Syndrome
- Twin-twin transfusion syndrome
References
- ↑ 1.0 1.1 1.2 Larsen, William J. (2001). Human embryology (3. ed.). Philadelphia, Pa.: Churchill Livingstone. pp. 490. ISBN 978-0-443-06583-5.
- ↑ 2.0 2.1 Underwood, Mark A.; Gilbert, William M.; Sherman, Michael P. (May 2005). "Amniotic Fluid: Not Just Fetal Urine Anymore - Journal of Perinatology". Journal of Perinatology 25 (5): 341–348. doi:10.1038/sj.jp.7211290. PMID 15861199.
- ↑ 3.0 3.1 Underwood, Mark A; Gilbert, William M; Sherman, Michael P (24 March 2005). "Amniotic Fluid: Not Just Fetal Urine Anymore". Journal of Perinatology 25 (5): 341–348. doi:10.1038/sj.jp.7211290. PMID 15861199.
- ↑ Caroline, Nancy L. (1977-01-03). "Medical Care in the Streets". JAMA: The Journal of the American Medical Association 237 (1): 43–6. doi:10.1001/jama.1977.03270280045020. ISSN 0098-7484. PMID 576129.
- ↑ Forewaters and hindwaters in Q&A section at babyworld.co.uk
- ↑ Koos, Brian J.; Rajaee, Arezoo (2014). "Fetal Breathing Movements and Changes at Birth". in Zhang, Lubo; Ducsay, Charles A. (in en). Advances in Fetal and Neonatal Physiology. Advances in Experimental Medicine and Biology. 814. New York, NY: Springer. pp. 89–101. doi:10.1007/978-1-4939-1031-1_8. ISBN 978-1-4939-1031-1. https://link.springer.com/chapter/10.1007/978-1-4939-1031-1_8.
- ↑ Koos, Brian J.; Rajaee, Arezoo (2014), "Fetal Breathing Movements and Changes at Birth", Advances in Fetal and Neonatal Physiology (Springer New York) 814: pp. 89–101, doi:10.1007/978-1-4939-1031-1_8, ISBN 978-1-4939-1030-4, PMID 25015803
- ↑ 8.0 8.1 Vaginal pH Test from Point of Care Testing, July 2009, at: University of California, San Francisco – Department of Laboratory Medicine. Prepared by: Patricia Nassos, PhD, MT and Clayton Hooper, RN.
- ↑ Bennett, S.; Cullen, J.; Sherer, D.; Woods Jr, J. (2008). "The Ferning and Nitrazine Tests of Amniotic Fluid Between 12 and 41 Weeks Gestation". American Journal of Perinatology 10 (2): 101–104. doi:10.1055/s-2007-994637. PMID 8476469.
- ↑ Meller, Daniel; Pauklin, Mikk; Thomasen, Henning; Westekemper, Henrike; Steuhl, Klaus-Peter (2011). "Amniotic Membrane Transplantation in the Human Eye". Deutsches Ärzteblatt International 108 (14): 243–248. doi:10.3238/arztebl.2011.0243. ISSN 1866-0452. PMID 21547164.
- ↑ Rennie, Kerry; Gruslin, Andrée; Hengstschläger, Markus; Pei, Duanqing; Cai, Jinglei; Nikaido, Toshio; Bani-Yaghoub, Mahmud (2012). "Applications of Amniotic Membrane and Fluid in Stem Cell Biology and Regenerative Medicine". Stem Cells International 2012. doi:10.1155/2012/721538. ISSN 1687-966X. PMID 23093978.
- ↑ Frank, Rachel M.; Cole, Brian J. (2018-11-22) (in en). OrthoBiologics in Sports Medicine, An Issue of Clinics in Sports Medicine, E-book. Elsevier Health Sciences. ISBN 978-0-323-65495-1. https://books.google.com/books?id=ZWp7DwAAQBAJ.
- ↑ "Stem cells in amniotic fluid show promise", Los Angeles Times, Jan 8 2007, retrieved 27 July 2009
- ↑ De Coppi, Paolo; Bartsch, Georg; Siddiqui, M Minhaj; Xu, Tao; Santos, Cesar C.; Perin, Laura; Mostoslavsky, Gustavo; Serre, Angéline C. et al. (2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biotechnology 25 (1): 100–106. doi:10.1038/nbt1274. PMID 17206138.
- ↑ "Scientists See Potential In Amniotic Stem Cells", Washington Post, Jan 8 2007, retrieved 27 July 2009
- ↑ "Amniotic Fluid Yields New Type of Stem Cell" , PBS - The Online News Hour, Jan 8 2007, retrieved 27 July 2009
- ↑ "Versatile Stem Cell Identified in Amniotic Fluid", Pamela J. Hines, International Society of Stem Cell Research, March 21, 2008, retrieve 27 July 2009 "ISSCR :: Public: Stem Cell Briefings". http://www.isscr.org/public/briefings/amniotic.htm.
- ↑ "Amniotic Stem Cells - "Mesenchimal Stem Cells in Human Application", Biocell Center Group, 2009, retrieved 27 July 2009 "Archived copy". http://www.biocellcenter.it/repository/scentific_review.pdf.
- ↑ De Coppi, Paolo; Bartsch, Georg; Siddiqui, M Minhaj; Xu, Tao; Santos, Cesar C.; Perin, Laura; Mostoslavsky, Gustavo; Serre, Angéline C. et al. (2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biotechnology 25 (1): 100–106. doi:10.1038/nbt1274. PMID 17206138.
 |
